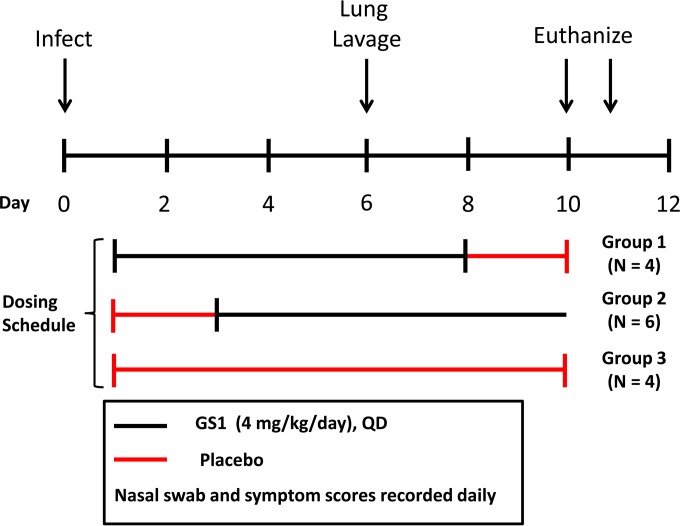
FIG 3.
Experimental design for study 2. Fourteen calves were inoculated with nebulized bRSV on study day 0. Group 1 (4 animals) was administered GS1 at 4 mg/kg/day once per day starting on study day 1 and continuing for 7 days, followed by administration of vehicle for 2 days. Group 2 (6 animals) was treated with vehicle for 2 days starting on study day 1, followed by administration of GS1 at 4 mg/kg once per day for 7 days beginning on study day 3. Group 3 (4 animals) received 9 consecutive vehicle treatments starting on study day 1. Blood samples were taken for plasma isolation from 3 calves (2 from group 1 and 1 from group 3) on study day 1 and 3 calves from study day 3 (2 from group 2 and 1 from group 3) to quantify drug levels. Nasal swabs and a blood sample were taken in the morning prior to dosing of each animal to measure viral load and drug concentration, respectively. Physical exams were conducted daily for the duration of the study to calculate the clinical symptom scores. On study day 6, selected animals that appeared in reasonably good health underwent bronchial lavage. The animals were euthanized on study day 10 or 11, and tissues were collected for necropsy and histopathology.