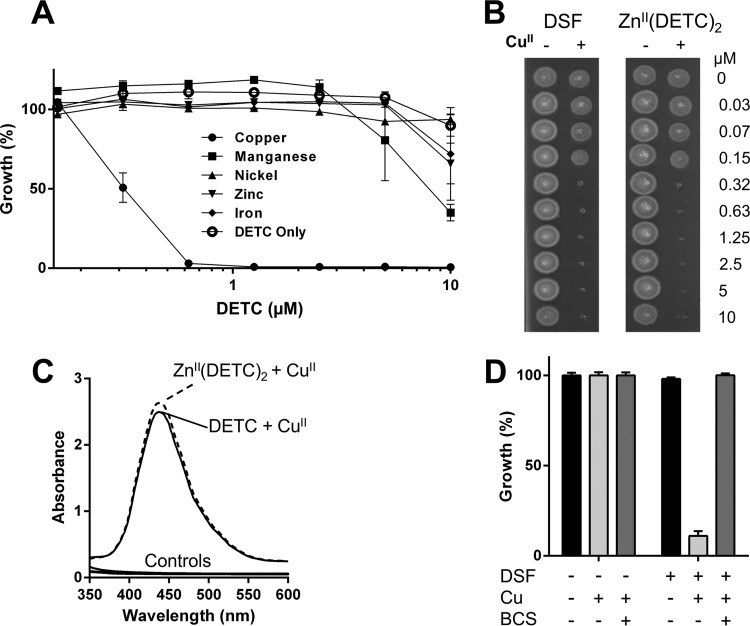
FIG 3.
Copper-dependent antimycobacterial effects of disulfiram. (A) Against M. tuberculosis mc26230, DETC displayed significant antimycobacterial effects only in the presence of CuII, but not if other tested physiologically relevant transition metals (CoII, FeII, NiII, MnII, or ZnII) were provided instead. (B) A commercially obtained zinc-DETC complex had no inhibitory activity against M. tuberculosis mc26230, but the addition of copper to the ZnII(DETC)2 complex resulted in an inhibition profile identical to that for disulfiram in the presence of copper. (C) A CuII(DETC)2 complex has a spectrophotometric peak at ∼435 nm; complex formation was observed even when commercially purchased ZnII(DETC)2 was used as the DETC source, indicating that copper can outcompete other complexed metals. Control spectra, consisting of DMSO, copper, and DETC alone, as well as the ZnII(DETC)2 complex in the absence of copper, are displayed; none displayed absorbance over the given spectrum. (D) While M. tuberculosis mc26230 is susceptible to as little as 0.3 μM disulfiram in 0.3 μM Cu (Fig. 2D), addition of 10 μM bathocuproine (BCS), a membrane-impermeable copper chelator, completely ablates activity.