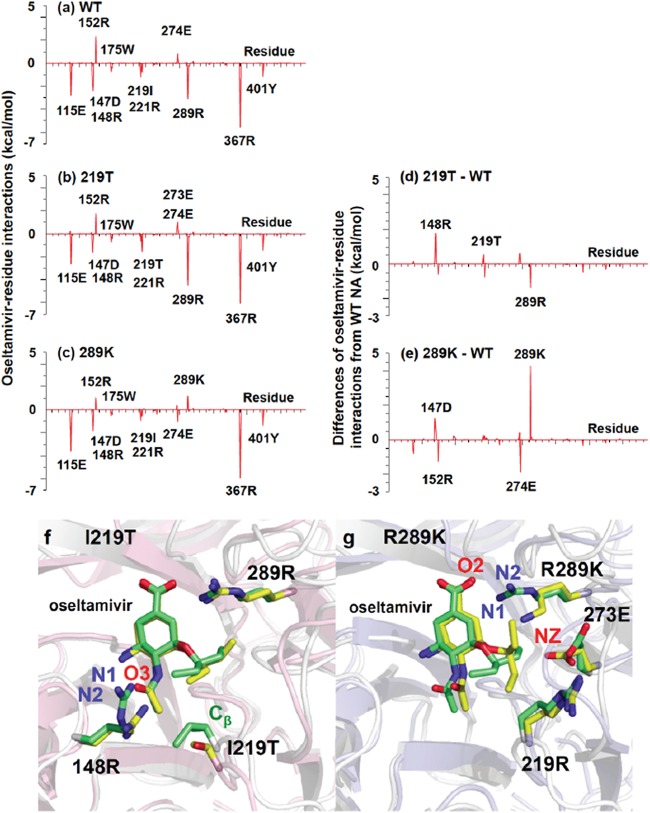
FIG 3.
Binding free energies between oseltamivir and N9 neuraminidase with substitutions at individual residues and simulated molecular structures of NA with T at 219 or K at 289 and oseltamivir. (a to c) Binding free energies between oseltamivir and individual amino acid residues (vertical axes) were calculated using the MM/GBSA free energy decomposition procedure. Residue numbers of amino acids in horizontal axes are indicated as residues in Anhui/1 N9 numbering. (a) Wild-type N9. (b) N9 with 219T. (c) N9 with 289K. (d and e) Changes of binding free energies resulting from amino acid substitution (vertical axes) were calculated by subtraction of the value determined for wild-type N9 from those determined for N9 with mutations. (d) N9 with 219T − wild-type N9 (= b − a). (e) N9 with 289K − wild-type N9 (= c − a). (f and g) Snapshots of simulated structures of the binding pockets in NA and oseltamivir were taken at 50 ns. Oseltamivir is shown in the middle of the figures. (f) Overlay of wild-type N9 (green) and N9 with 219T (Anhui N9 numbering) (yellow). (g) Overlay of wild-type NA (green) and N9 with 289K (yellow).