FIGURE 10.
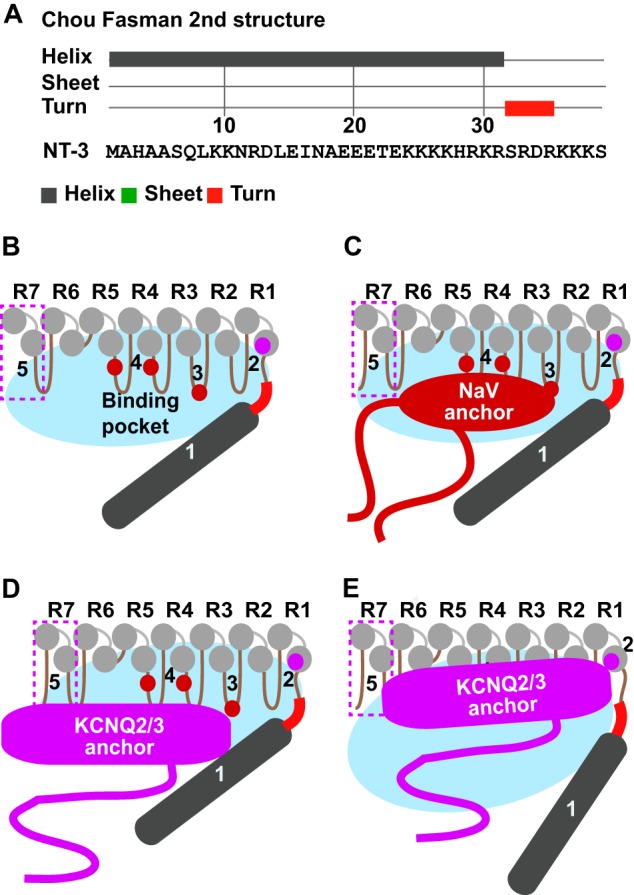
Gated binding pocket model for differential Nav and KCNQ2/3 channel binding to AnkG. A, Chou-Fasman secondary structure algorithm predicts the NT3 N terminus forms an α-helix (gray) followed by a three-four-residue turn (red) linked to downstream ankyrin repeats. B–E, model summarizing results of the current study, also considering results of Wang et al. (40). Five determinants of binding are shown: 1, the N terminus; 2, repeat 1 helix RAAR; 3, hairpin 3 KK; 4, two F residues on hairpins 4 and 5 (40); 5, repeat 7. The non-ankyrin N-terminal (dark gray) together with ankyrin repeats R1–7 (light gray) surround a binding pocket (blue oval). In the preferred “tight” conformation of the pocket (B–D), the smaller Nav anchor domain accesses its binding sites more easily than the larger KCNQ2/s3 anchor domain.
