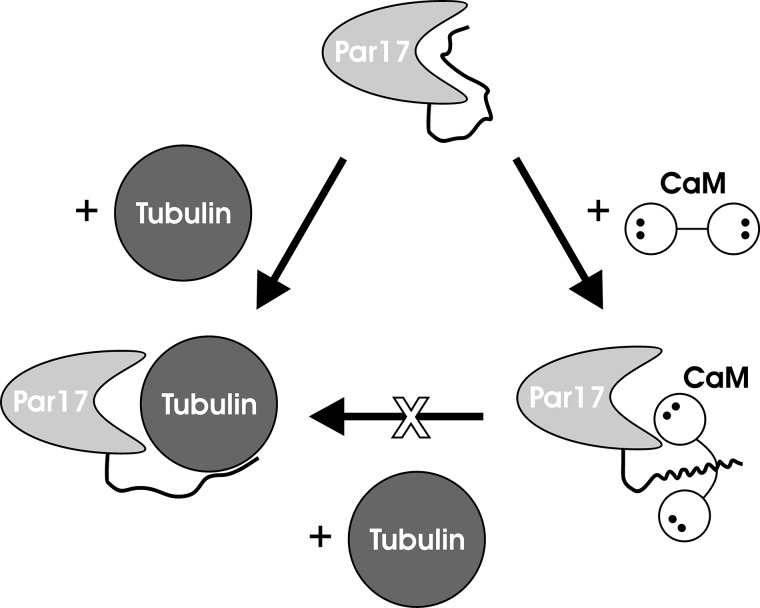
FIGURE 11.
Schematic representation of the Ca2+/CaM-dependent blocking of the Par17-promoted tubulin polymerization. In native Par17 (top), the N-terminal extension interacts with the substrate binding pocket. In the tubulin-Par17 complex (bottom left), tubulin seems to continue to interact with the Par17 N terminus after replacing it at the substrate binding pocket, thus ensuring a higher polymerization efficiency compared with Par14. Ca2+/CaM binds strongly to the Par17 N terminus (bottom right); at the same time, the close proximity of CaM to the substrate binding pocket of the PPIase domain apparently restricts the access to the active site for larger ligands such as tubulin but not for small substrates such as the oligopeptide succinyl-AKPF-4-nitroanilide.