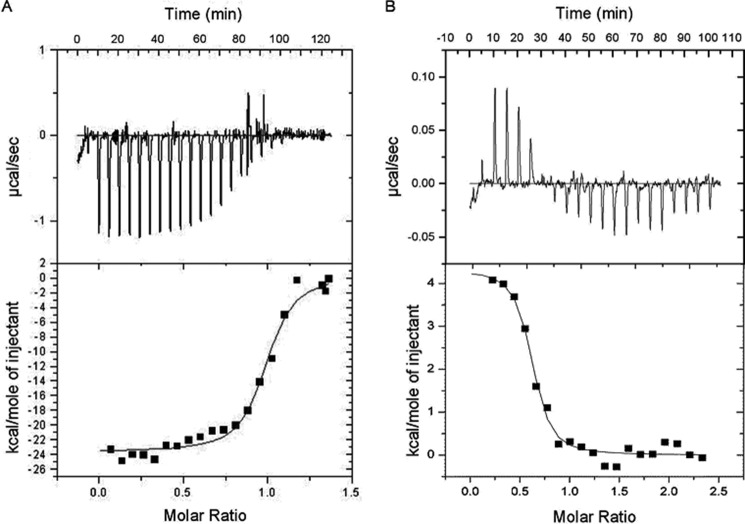
FIGURE 3.
ITC data of Ca2+/CaM binding to the Par17 N terminus. The calorimetric trace of heat released upon titration of Ca2+/CaM into a solution containing either the Par172–22 peptide (A) or full-length Par17 (B) is shown at the top. The corresponding heat per mole of injected Ca2+/CaM is shown at the bottom. The binding isotherms were analyzed by applying a single-site binding model using the Microcal Origin software package. The analyses revealed the following thermodynamic parameters for the Par172–22 peptide (ΔH = −23.6 ± 0.4 kcal/mol, ΔS = −49.2 cal/(mol·degree), n = 0.97 ± 0.01, Kd = 149 ± 25 nm) and for the full-length Par17 protein (ΔH = 4.3 ± 0.2 kcal/mol, ΔS = 45.8 cal/(mol·degree), n = 0.59 ± 0.02, Kd = 143 ± 41 nm).