FIGURE 2.
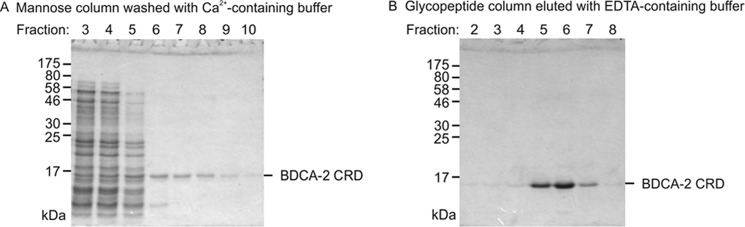
Purification of the CRD from BDCA-2 by affinity chromatography. A, the CRD was renatured from inclusion bodies by dialysis against Ca2+-containing buffer followed by dialysis against water and lyophilization to obtain a concentrated sample that was applied to a 10-ml column of mannose-Sepharose. After application of the sample, the column was eluted with 150 mm NaCl, 25 mm Tris-Cl, pH 7.8, 25 mm CaCl2. Fractions of 2 ml were collected. B, following renaturation by dialysis, the CRD was applied directly to a 5-ml column of desialylated egg yolk glycopeptide immobilized on agarose. The column was rinsed with 25 ml of 150 mm NaCl, 25 mm Tris-Cl, pH 7.8, 25 mm CaCl2, and protein was eluted with 150 mm NaCl, 25 mm Tris-Cl, pH 7.8, 2.5 mm EDTA in 1-ml fractions. In both cases, aliquots (15 μl) from fractions were analyzed on SDS-polyacrylamide gels that were stained with Coomassie Blue. The expected molecular weight of the BDCA-2 CRD is 17,100.
