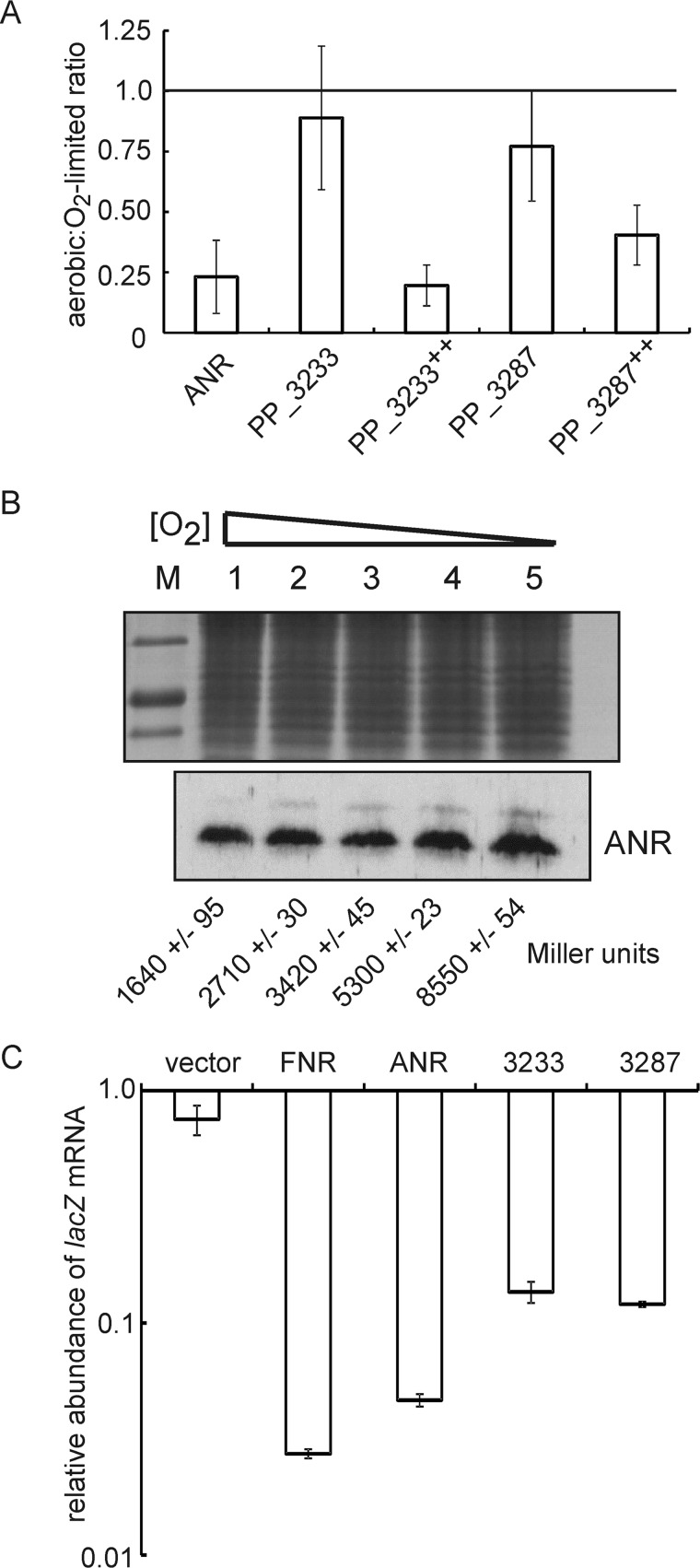
FIGURE 7.
Responses of P. putida FNR proteins to O2in vivo. A, the output from an FNR-dependent promoter decreases in response to enhanced aeration of P. putida cultures expressing only one of the three FNR proteins. All the strains were transformed with the FF-41.5-lacZ reporter plasmid pGS810. The rate of culture aeration was increased by decreasing the volume of medium in the shaking conical flasks (50 ml of medium for O2-limited cultures and 10 ml of medium for aerobic cultures). Cultures were grown at 30 °C for 3 h, at which point samples were taken for measurement of β-galactosidase activity. The β-galactosidase activities of the aerobic cultures were divided by those of the O2-limited cultures. The error bars are the standard deviation from the mean values of the aerobic:O2-limited ratios (n = 4). ANR, PP_3233, and PP_3287 indicate chromosomal expression of the corresponding genes; PP_3233++ and PP_3287++ indicate expression of the corresponding genes from a multicopy plasmid. B, concentration of cytoplasmic ANR does not respond to changes in culture aeration. Shown are Western blots developed with anti-serum raised against E. coli FNR for cell samples from P. putida cultures that express only anr grown in shaking 50-ml conical flasks containing 10, 20, 30, 40, or 50 ml of medium (lanes 1–5) to impose an increasing degree of O2 limitation on the cultures. The equivalent region of a Coomassie Blue-stained gel is shown as a loading control (M indicates protein standard markers: 37, 25, and 20 kDa, top to bottom). The outputs from the pFF-41.5 reporter (pGS810) for cultures grown as described above are shown below each lane (mean values ± standard deviation, n = 3). C, inactivation of FNR proteins upon exposure of anaerobic cultures to air. Cultures of E. coli JRG6348 expressing either no FNR (vector), E. coli FNR, P. putida ANR, PP_3233, or PP_3287, as indicated, were grown under anaerobic conditions and the abundance of FNR-protein-dependent lacZ transcription was measured by qRT-PCR. The cultures were exposed to air for 20 min, and then the abundance of the lacZ transcript was measured again. The relative abundance of lacZ mRNA after transfer to aerobic conditions is shown. The error bars are the standard deviation from the mean (n = 3).