FIGURE 2.
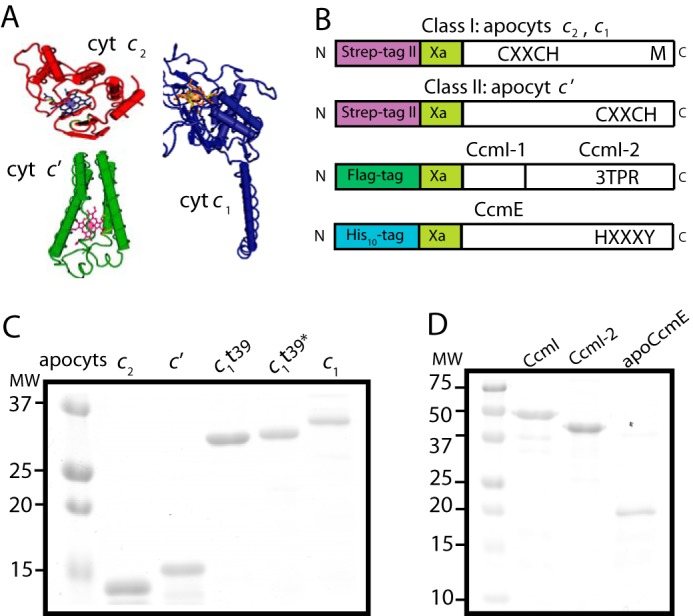
Purification of various R. capsulatus c-type apocytochromes, and CcmI and apoCcmE. A, three-dimensional structures of class I cytochrome c2 (Cyt c2; Protein Data Bank code: 1C2R), cytochrome c1 (Cyt c1; Protein Data Bank code: 1ZRT), and class II cytochrome c′ (Cyt c′; Protein Data Bank code: 1RCP). B, schematic representations of c-apocytochrome constructs with N-terminal Strep-tag followed by a Factor Xa cleavage site. The heme-binding site, C1XXC2H, is located close to the N and C termini in class I and class II c-type apocytochromes, respectively. The sixth axial ligand (M) in class I c-type apocytochromes is located at the C terminus, whereas in class II, apocytochrome c′ heme has no sixth axial ligand. CcmI, formed from the CcmI-1 and CcmI-2 (with its TPR motifs) domains, has an N-terminal FLAG tag followed by a Factor Xa cleavage site. ApoCcmE (with its HXXXY heme binding motif) has an N-terminal His10 tag fused at its membrane anchor. C, Coomassie Blue-stained SDS-PAGE containing 3 μg of Strep-Tactin Sepharose-purified Strep-apocytochrome c2 (lane 1), Strep-apocytochrome c′ (lane 2), Strep-apocytochrome c1t39 (lane 3), Cys-less derivative of Strep-apocytochrome c1t39* (lane 4), and Strep-apocytochrome c1 (lane 5). t39, refers to the truncation of 39 C-terminal amino acid residues encompassing the membrane anchor of cytochrome c1. D, Coomassie Blue-stained SDS-PAGE containing 3 μg of Anti-FLAG Sepharose-purified CcmI (lane 1), Ni-Sepharose-purified His10-CcmI-2 (lane 2), and apoCcmE (lane 3).
