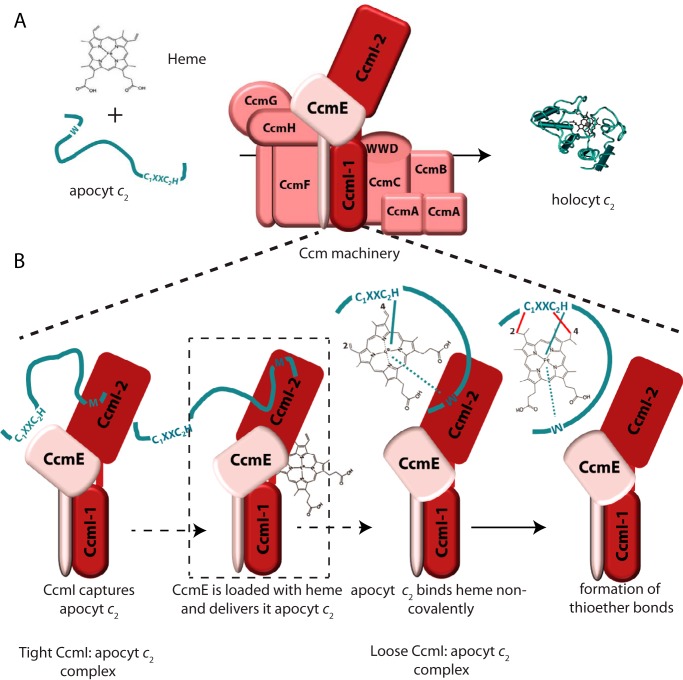
FIGURE 9.
CcmI-apocytochrome c2 interactions in the absence and presence of hemin during the Ccm process. A, shows the Ccm machinery and its two substrates, apocytochrome c2 and heme, forming holocytochrome c2. B, depicts the interactions between CcmI and apocytochrome c2 in the absence and presence of hemin (thought to be provided by CcmE). This hypothetical scheme takes into account all available data to show that the CcmI-2 portion of CcmI binds tightly the C-terminal helix of apocytochrome c2, bringing its heme-binding site near CcmE (left panel). Upon the availability of heme via CcmE, apocytochrome c2 binds heme noncovalently to form a b-type cytochrome intermediate that interacts less tightly with CcmI-2 (middle two panels). The subsequent formation of covalent thioether bonds between heme and apocytochrome c2, via currently unknown steps catalyzed by the remaining components of Ccm System I, yields holocytochrome c2 (right panel).