Abstract
Sphingolipid synthesis involves a highly conserved biosynthetic pathway that produces fundamental precursors of complex sphingolipids. The final reaction involves the insertion of a double bond into dihydroceramides to generate the more abundant ceramides, which are converted to sphingomyelins and glucosylceramides/gangliosides by the addition of polar head groups. Although ceramides have long been known to mediate cellular stress responses, the dihydroceramides that are transiently produced during de novo sphingolipid synthesis were deemed inert. Evidence published in the last few years suggests that these dihydroceramides accumulate to a far greater extent in tissues than previously thought. Moreover, they have biological functions that are distinct and non-overlapping with those of the more prevalent ceramides. Roles are being uncovered in autophagy, hypoxia, and cellular proliferation, and the lipids are now implicated in the etiology, treatment, and/or diagnosis of diabetes, cancer, ischemia/reperfusion injury, and neurodegenerative diseases. This minireview summarizes recent findings on this emerging class of bioactive lipids.
Keywords: apoptosis, autophagy, cell signaling, hypoxia, membrane, ceramides, sphingolipids, dihydroceramide, proliferation
Introduction
“Remember: there are no small parts, only small actors.” Konstantin Stanislavsky (Russian Actor, 1863–1938).
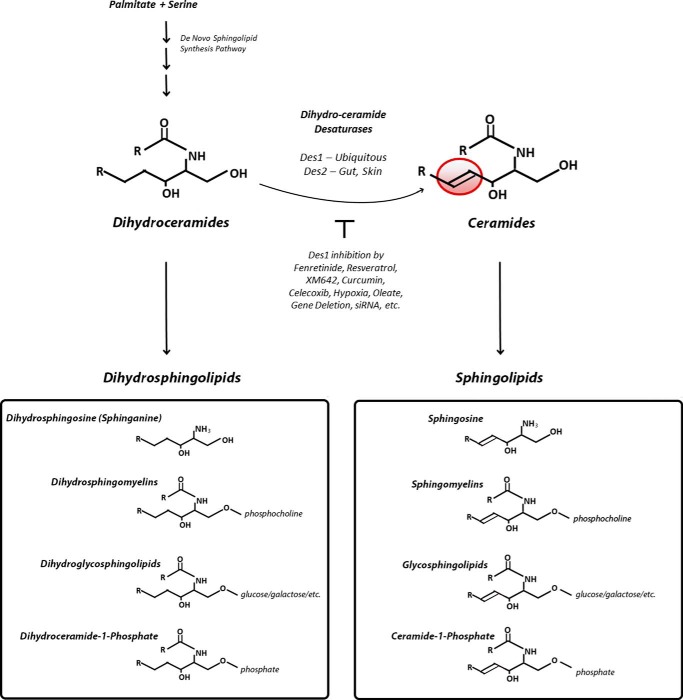
Sphingolipids are the second largest class of membrane lipids, and thousands of distinct species have been identified (see the LIPID MAPS Lipidomics Gateway). They have a diverse array of functions related to cell survival, membrane integrity, metabolic regulation, and general adaptations to cellular stressors. Despite the incredible complexity of the sphingolipid pool, their production relies on a simple and highly conserved four-step de novo synthesis pathway (1). The final reaction involves the insertion of a double bond into dihydroceramide (Fig. 1) to convert it to ceramide, the precursor for the preponderant complex sphingolipids.
FIGURE 1.
Schematic depicting the importance of the DES1 reaction in de novo ceramide biosynthesis.
Two distinct dihydroceramide desaturases (DES1 and DES2)3 catalyze the desaturation reaction, with DES1 accounting for ceramide synthesis in most tissues (2, 3). By contrast, DES2, which also contains c-4 hydroxylase activity and thus also generates phytoceramides, is prevalent in skin, intestine, and kidney. Much of the work implicating dihydroceramides or other complex dihydrosphingolipids in biology involves pharmacological inhibition or ablation of the genes/mRNAs encoding these endoplasmic reticulum-resident proteins to elevate levels of the endogenous lipids.
The double bond that distinguishes dihydroceramides from ceramides markedly alters the biophysical properties of the molecules, modifying their elastic properties and packing behavior (4). Like ceramides, dihydroceramides are also precursors for complex sphingolipids (e.g. dihydrosphingomyelin and dihydroglucosylceramides/dihydrogangliosides) (Fig. 1) (5). Unlike ceramides, they are far less abundant, and were for years deemed inert. In an early study demonstrating that ceramide was bioactive, Hannun and co-workers (6, 7) found that the desaturation of sphingolipid metabolites through the introduction of the double bond altered their function, as short-chain analogs of ceramide were able to induce apoptosis and block cell growth, whereas analogs of dihydroceramides were ineffective. Multiple other groups, including our own, used these water-soluble analogs to come to the conclusion that the dihydro-forms of the lipids were ineffectual in a wide range of biological responses such as platelet aggregation (8), cell growth (9), DNA damage (10), regulation of ion channels (11, 12), and inhibition of insulin signaling and glucose uptake (13). As a result, the mistaken dogma emerged that dihydroceramides are biologically inactive.
A critical breakthrough revealing that dihydroceramides might have regulatory roles in biology came from an unlikely discovery from the Merrill group (14). They profiled the sphingolipidome of cells treated with fenretinide (N-(4-hydroxyphenyl)retinamide), a vitamin A analog with chemotherapeutic properties. The compound has been widely studied and is in clinical trials for treatment of various cancer pathologies and glucose intolerance. The drug had been thought to induce ceramide, a presumed mechanism by which it might induce apoptosis, slow proliferation, and therefore halt tumor growth. However, the application of refined mass spectroscopic techniques revealed that it was in fact the precursor dihydroceramides and other dihydroceramide-containing sphingolipids that accrued. By contrast, ceramide-derived sphingolipids containing the double bond in the sphingoid moiety were unchanged or reduced. Subsequent studies revealed that fenretinide, which bears a structural resemblance to dihydroceramides, irreversibly inhibits DES by disrupting the electron transport necessary for the desaturation event (15). Using both fenretinide and dihydroceramide analogs, the Merrill laboratory (14) identified a likely role in the induction of autophagy (see below). This study inspired a number of groups to evaluate the role of dihydroceramides in cellular responses, and these sphingolipids are now implicated in a broad cadre of biological processes.
Autophagy
Autophagy is a catabolic mechanism through which cells degrade organelles and recycle them into macromolecules that serve as cellular constituents or metabolic fuels. The process involves the isolation of organelles from the rest of the cell within a double-membrane vesicle that subsequently fuses with lysosomes. The process is important for surviving harsh conditions (e.g. starvation) as well as in normal biology (e.g. development). Numerous groups have implicated autophagy in cell death (16, 17), although this remains controversial (18).
Autophagy was one of the first biological responses assigned to dihydroceramides, as both fenretinide and exogenous, short-chain (C2) dihydroceramides induced formation of autophagosomes (14). Subsequent studies involving other pharmacological inhibitors to reduce DES1 activity (19, 20) or using fibroblasts from knock-out mice lacking the first exon of the Des1 gene (21) further support a role for these unique sphingolipids in autophagy induction. In all of these studies, inhibition of DES1 increased sensitivity to autophagic stimuli. By contrast, inhibition of enzymes upstream of Des1 (i.e. in macrophages treated with TLR4 agonists) blocks autophagy induction (22). In all of these studies, neither dihydroceramide accumulation nor the persistent autophagy contributed to cell death, as desaturase inhibition instead conferred resistance to apoptosis (see below).
The precise mechanisms linking dihydroceramides to autophagy are unresolved, but clues emerged from a study showing reduced rates of ATP synthesis in embryonic fibroblasts from Des1 knock-out mice (21). Mechanistically, this results from impaired electron transport chain activity attributable to the ability of one or more dihydrosphingolipids to disrupt activity of components of the electron transport chain. Treatment with myriocin, an inhibitor of the first enzyme in the dihydroceramide/ceramide biosynthetic cascade, restored rates of ATP synthesis in these cells, confirming that the accumulation of the dihydrosphingolipids likely drove the response, rather than the absence of ceramides. The compromised ability of the cell to generate energy in nutrient-replete conditions leads to activation of AMP-activated protein kinase, and the addition of ATP or knockdown of AMP-activated protein kinase reversed the autophagic phenotype. Amazingly, the rampant autophagy in these knock-out cells occurred despite a marked up-regulation of the Akt/mTOR (mammalian target of rapamycin) pathway, which is potently anti-autophagic. This latter effect of DES1 likely resulted from the depletion of ceramides, which are known to block activation of Akt. Indeed, myriocin failed to affect the Akt/mTOR pathway.
Hypoxia
Hypoxia refers to a state where the body or tissues receive inadequate supplies of oxygen, such as during high altitude climbing or reduced blood delivery during ischemia. Most organisms show an immediate response to a hypoxic environment and quickly die in an anoxic one.
Devlin et al. (23) profiled sphingolipids in the lungs of rats subjected to hypoxia, noting a rapid, time-dependent up-regulation of dihydroceramides. The increase was proportional to the depth and duration of the hypoxic insult. By contrast, oxygen levels did not impact levels of ceramides, and the change was restricted to sphingolipids lacking the double bond introduced by DES1. The inhibition of cell proliferation seen in hypoxia was reproduced by knockdown of either DES isoform in cultured cells, even when cells were maintained in oxygen-replete conditions. Conversely, overexpression of either desaturase prevented the hypoxic effects, and the group implicated DES1 and DES2 as oxygen sensors. Similar findings are reported in the mouse heart and cultured cells, which show reduced levels of ceramides and accumulation of dihydroceramides as they adapt to a hypoxic environment (24).
The mechanisms through which dihydroceramides mediate biological responses to hypoxia are unclear, although the effect appears to be independent of the hypoxia-inducible transcription factors. Nonetheless, numerous other studies reveal that dihydroceramides slow cell proliferation (see below), which is a common cellular response to oxygen depletion.
Several mechanisms have been proposed to explain how cellular oxygen levels inhibit dihydroceramide desaturase activity to induce dihydroceramides. The response is rapid, and DES enzymes are directly activated by oxygen (23, 25). Oxygen is present at the core of the Des1 enzyme and is requisite for formation of an “oxo bridge” with iron, which is needed because Des1 utilizes oxygen in the desaturase reaction (25). Based on analogy with oxygen-dependent prolyl-hydroxylases, Devlin et al. (23) advanced a proposal that Des1 fits the criteria as a direct oxygen sensor.
Levels of transcripts encoding DES1 also decrease in hypoxia, suggesting a potential transcriptional mechanism. The effect on mRNA levels is independent of hypoxia-inducing factor-1α 1 (23), but might be explained through HAND2 and nuclear factor of activated T-cells (NFATC) transcription factors (24), for which the DES1 promoter harbors binding sites (24).
Of note, Menuz et al. (26) used Caenorhabditis elegans to screen for novel effectors of the hypoxic response. Unlike most organisms, C. elegans can survive a completely anoxic environment for at least 48 h. Loss of the (dihydro)ceramide synthase gene Hyl-2 actually opposed their sensitivity to oxygen deprivation.
Considerable work remains on this interesting topic, but roles for dihydroceramides in this response could identify the desaturase as a therapeutic target for mitigating tissue responses to hypoxia, such as in ischemic injury during stroke and acute myocardial infarction. Rodrigues-Cuenca et al. (27) additionally suggested that the subsequent activation of DES1 during cardiac reperfusion could contribute to the damage seen, resulting from sudden increases in de novo ceramide production.
Cell Proliferation
Experimental interventions in cultured cells that inhibit DES1 activity generally inhibit cell proliferation. For example, the inhibitory effects of hypoxia on BrdU accumulation in DNA can be recapitulated by DES1 knockdown and reversed by DES1 overexpression (23). Moreover, transfection of SMS-human neuroblastoma cells (SMS-KCNR) cells with small interfering RNA to the transcript encoding DES1 promoted accumulation of endogenous dihydroceramides, inhibited cell growth, and induced cell cycle arrest at G0/G1. This was accompanied by a significant decrease in the amount of phosphorylated retinoblastoma protein (28). Embryonic fibroblasts isolated from DES1 knock-out mice also proliferate slowly (21). Nonetheless, the mechanisms through which dihydroceramides control rates of cell division remain ambiguous.
Studies with various anti-tumor compounds or pharmacological reagents also suggest roles for dihydroceramides in the regulation of cell proliferation (29). (a) Curcumin, isolated from the dietary spice turmeric, induces G2/M cell cycle arrest and autophagy, but not apoptosis, in malignant glioma cells. It also blocks tumor growth in vivo. Curcumin inhibits DES1 activity at an IC50 below 25 μm (reviewed in Ref. 29). (b) The cyclooxygenase-2 inhibitor celecoxib promotes dihydroceramide accumulation while depleting cells of ceramide. Celecoxib inhibits DES1 activity in intact cells at an IC50 of about 80 μm. Blocking dihydroceramide production with an inhibitor of the first enzyme in the synthesis pathway (i.e. myriocin, which inhibits serine palmitoyltransferase) reversed the anti-proliferative potency of celecoxib (reviewed in Ref. 29), suggesting that sphingolipids were obligate intermediates in the drug response. (c) Brodesser and Kolter (81) also conducted studies using a dihydroceramide desaturase inhibitor, XM462, which induces a transient early increase in dihydroceramides and other sphingolipids composed of a dihydroceramide backbone. This dihydroceramide accumulation was also associated with decreased proliferation rates and a delayed G1/S transition (19).
Pathogen-derived Phospho-dihydroceramides in Innate Immunity
The innate immune system defends plants and animals from infection against multiple invading pathogens. The process involves eosinophils, monocytes, macrophages, and natural killer cells and utilizes Toll-like receptors (TLRs) that recognize endotoxins such as lipopolysaccharide or lipid A. The response differs from adaptive immunity present in higher eukaryotes, which is slower but more specific for the particular biological insult. Recent studies have implicated dihydroceramide derivatives in the innate immune response underlying periodontal disease.
Nichols et al. (30–32) quantified sphingolipids in the periodontal pathogen Porphyromonas gingivalis and found that phosphorylated dihydroceramides activated antigen-producing cells to secrete cytokines. The proposed mechanism is that these unique bacterial lipids serve as direct ligands for TLR2, and the group took care to demonstrate that this effect was independent of contaminating LPS (30–32). These bacterial lipids could be recovered from diseased human tissue samples and blood, implicating phosphorylated dihydroceramides in the peripheral immune response associated with this condition (33).
HIV Infection
HIV infection requires the fusion of the viral and host membranes, driven by a peptide domain of the HIV gp41 protein. The insertion depth of this moiety is an important determinant of fusogenic capabilities (34). Vieira et al. (35) discovered that knockdown or pharmacological inhibition of DES1, which replaced sphingomyelins with dihydrosphingomyelins, inhibited infection by replication-competent and -deficient HIV-1 in cultured cells. The increased dihydrosphingolipid levels gave rise to more rigid membranes that were resistant to the insertion of the gp41 fusion peptide, thus inhibiting fusion of the viral and cellular membranes. These results clarified the effect of the double bond in membrane fluidity and identified DES1 as a potential therapeutic target for combating HIV-1 infection.
Attenuation of Ceramide-induced Apoptosis
Apoptosis is a process of programmed cell death characterized by membrane blebbing, cell shrinkage, nuclear fragmentation, chromatin condensation, and chromosomal DNA fragmentation. Ultimately, the events result in production of cell fragments that are engulfed by phagocytosis. This form of cell death differs from necrosis, which is a traumatic form of cell death that results from acute cellular injury, resulting in the release of harmful metabolites into the circulation. Apoptosis is implicated in normal physiology as well as various disease processes.
Early in apoptosis, the mitochondrial outer membrane becomes permeable to small proteins. The release of cytochrome c from the organelle activates a cysteine-dependent, aspartate-directed protease (caspase 9), which triggers cleavage and activation of other caspases, which regulate and degrade several additional proteins.
The role of dihydroceramides in apoptosis is controversial. Some groups have implicated dihydroceramides in the induction of apoptosis, and both fenretinide and resveratrol induce apoptosis in transformed cell lines (20, 36–38). Moreover, inhibitors of enzymes upstream of DES1 in the de novo synthesis pathway have been shown to reverse the apoptotic effects of fenretinide in endothelial cells (36, 39). Nonetheless, studies in other cell types fail to reveal a critical role for sphingolipids in fenretinide-induced apoptosis (40), and the preponderance of data suggests that the sphingolipid is insufficient to induce apoptosis or cell death. Inhibiting cellular DES1 activity with other pharmacological reagents, siRNA, or gene depletion renders cells strongly resistant to apoptosis caused by diverse stimuli (19, 21, 41, 42).
In contrast to dihydroceramides, roles for ceramides generated from either de novo synthesis and/or sphingomyelinase activation in cytochrome c release or apoptosis induction are firmly established. Mechanisms include the regulation of Bcl2 family members (43, 44), the formation of ceramide channels in mitochondrial membranes (45–47), inhibition of the pro-survival AKT/PKB kinase (48), the clustering of death receptors in the plasma membrane (49–52), and others (53, 54). Many of the signaling effects of ceramide were initially attributed to activation of a protein phosphatase or protein kinase Cζ (55), although signaling roles for the sphingolipid have long been challenged (56). Regardless, other mechanisms almost certainly contribute to a ceramide-induced cell death.
Evidence does suggest that dihydroceramides may counter ceramide-induced cell death. For example, knockdown of DES1 in human head and neck squamous carcinoma cells attenuated apoptosis induced by photodynamic therapy (42). Mitochondrial depolarization, late apoptosis, and cell death were all attenuated by DES1 knockdown. The treatment increased levels of dihydroceramides without affecting cellular ceramide levels. One mechanism may be through the regulation of the aforementioned ceramide channels. Dihydroceramides disrupt formation of ceramide pores in mitochondria (57). This inhibition occurs with very small concentrations of dihydroceramides, suggesting that ratios of the two lipids may be an important determinant in apoptosis induction.
Oxidative Stress
Reactive oxygen species (ROS) including oxygen ions and peroxides are chemically reactive intermediates produced by the normal aerobic metabolism of oxygen. Although implicated in signal transduction, they are most noted for their contribution to eukaryotic stress responses. For example, ROS damage DNA and oxidize polyunsaturated fatty acids (i.e. lipid peroxidation) and amino acids, leading to marked alterations in cellular function and organismal health.
Fenretinide has long been known to induce ROS (58–60). Moreover, inhibition of DES1 by either gene ablation or knockdown increases ROS severalfold.4 Nonetheless, Apraiz et al. (61) found that dihydroceramide was not essential for fenretinide-induced ROS production or cell death.
Dihydroceramide production is also a consequence of ROS generation, as the oxidative stressors hydrogen peroxide, menadione, or tert-butyl-hydroperoxide increase dihydroceramide levels in various immortalized cell lines (19). By contrast, ceramide levels were unaffected by these compounds. Mechanistic studies revealed that this was a result of changes in DES1 activity rather than changes in protein expression (62), perhaps resulting from depletion of thiol or changes in NADPH, a necessary cofactor for DES1 (62, 63).
Biomarkers of Metabolic Disorders
Obesity is a major risk factor for the development of type 2 diabetes and cardiovascular diseases, and much attention has been given to mechanisms linking increased adiposity to the development of these metabolic pathologies. The lipotoxicity hypothesis posits that the accumulation of fat-derived molecules contributes to tissue dysfunction such as the impairment of insulin action, induction of metabolic dysfunction, or death/apoptosis of cells that are essential for tissue function. Sphingolipids such as ceramide are likely contributors to lipotoxicity. The oversupply of saturated fatty acids to peripheral tissues provides palmitate, which is utilized by the sphingolipid synthesis pathway. Moreover, low level inflammation found in obesity selectively up-regulates sphingolipid biosynthesis, amplifying the effect of lipid oversupply.
The most compelling studies implicating sphingolipids in insulin resistance and other complications of obesity (e.g. cardiovascular disease) are interventional ones evaluating the consequences of inhibiting enzymes required for de novo sphingolipid synthesis on various metabolic disease endpoints. Most notably, inhibition of the first enzyme in the pathway (i.e. serine palmitoyl-transferase) in mice by administering a pharmacological inhibitor or deleting a gene allele renders mice resistant to insulin resistance, diabetes, and/or other cardiovascular complications of obesity (64–67).
Dihydroceramides have received recent attention as biomarkers of metabolic dysfunction. Lopez et al. (68) found elevated levels of circulating ceramides and one dihydroceramide in female children and adolescents with type 2 diabetes. More recently, Mamtani et al. (69) found a specific association between serum dihydroceramides and waist circumference in Mexican Americans. Brozinick et al. (70) found associations between circulating levels of dihydroceramides, as well as deoxyceramides and ceramides, with the severity of insulin resistance in non-human primates. Dihydroceramide levels often correlate more tightly than ceramide levels with various metabolic disease endpoints. Although this could suggest a causative role for the sphingolipid, the preponderance of evidence suggests otherwise (see below). An alternative explanation is that less abundant sphingolipids such as dihydroceramides may be predictive of metabolic disease, and we hypothesize that they may serve as a readout of rates of sphingolipid synthesis or flux.
Despite the correlative evidence revealing that dihydroceramides may indeed be markers of metabolic disease endpoints, interventional studies in mice or cultured cells have thus far failed to reveal a causative role for dihydroceramides in the induction of insulin resistance or hypertension. Indeed, inhibiting DES1 is generally protective. First, fenretinide improves insulin sensitivity and is in clinical trials assessing the potential of this compound in glucose-intolerant patients. Although the drug's efficacy was initially attributed to its ability to displace retinol from retinal-binding protein-4 (71), the compound can clearly increase insulin sensitivity and ameliorate the pathogenic consequences of obesity through alternative, RBP4-independent pathways (72, 73). Moreover, mice that are haploinsufficient for DES1 are protected from glucocorticoid-induced insulin resistance (67) and diet-induced hypertension (74). These studies identify ceramide-derived sphingolipids as those involved in lipotoxic events.
Studies in cultured cells further support the supposition that ceramides, but not dihydroceramides, antagonize insulin action. Analogs of ceramides, but not dihydroceramides, inhibit insulin signaling to AKT/PKB and its stimulation of glucose uptake (13). Using cultured myotubes, researchers found evidence that inhibition of DES1 prevented lipid-induced inhibition of insulin signaling to AKT/PKB. The addition of exogenous palmitate, which induces synthesis of both dihydroceramides and ceramides, antagonizes insulin activation of AKT/PKB. The lipid increases expression of transcripts encoding DES1, whereas knockdown of DES1 protects from palmitate inhibition of insulin signaling (75, 76). Interestingly, the monounsaturated fatty acid oleate decreases the activity of DES1 and prevents lipid-induced insulin resistance (73, 75).
Although dihydroceramides do not impact insulin signaling directly, more recent studies identified roles for dihydroceramides in adipose biology. The Vidal-Puig group (77) found reduced levels of DES1 mRNA in obese humans and mice. Moreover, they found that levels of the transcript increased during differentiation of adipocytes in culture. A 3T3-L1 preadipocyte cell line in which they stably knocked down DES1 displayed elevated oxidative stress, cell death, and apoptosis, as well as decreased proliferation. Moreover, the cells displayed markedly impaired adipogenesis. Pharmacological inhibition of DES1 recapitulated the anti-adipogenic effects in vitro and in vivo. By contrast, the genetic ablation of the Des1 homolog in flies promoted fat storage concomitantly with increased level of dihydroceramides (78).
Conclusion and Future Perspectives
A simple screen of the PubMed database with the search word dihydroceramide currently unveils 528 studies. Many of these publications dealt with the characterization and regulation of the desaturase, particularly as it relates to production of ceramides and induction of apoptosis. Indeed, throughout this study, we have highlighted evidence revealing regulatory effects of drugs (e.g. fenretinide, resveratrol, etc.) or biological stimuli (oxidative stress, hypoxia, fatty acids, etc.) on DES1/DES2 activity or expression. A body of evidence also suggests a role for DES1 myristoylation in the control of activity (44, 79, 80).
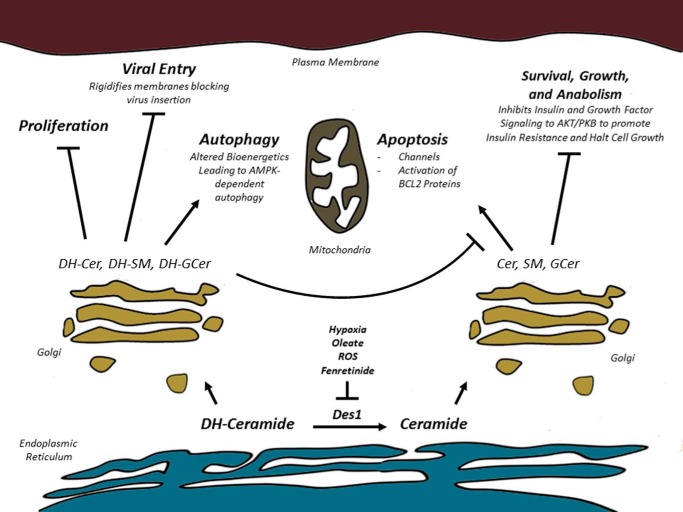
Although studies investigating the biological role are in their infancy, emerging data using multiple approaches reveal roles for dihydroceramides in autophagy and proliferation (Fig. 2). Emerging studies suggest additional roles in innate immunity, virus entry, and oxidative stress. In apoptosis and metabolic regulation, where causal roles for dihydrosphingolipids are less clear, control of the rate of conversion of dihydroceramide into ceramide is an important site of regulation.
FIGURE 2.
Schematic depicting the non-overlapping biological responses triggered by dihydroceramides versus ceramides. AMPK, AMP-activated protein kinase; DH-Cer, dihydroceramide; DH-SM, dihydrosphingomyelin; DH-GCer, dihydroglucosylceramide.
The mechanisms allowing cells to respond differently to sphingolipids containing the sphingosine versus sphinganine moiety is somewhat surprising because of the relatively small molecular change and the fact that this modification is imbedded in the membrane bilayer. Nonetheless, studies in knock-out mice clearly illustrate the importance of the desaturation reaction. In DES1 knock-out mice, the majority of sphingolipids lack the double bond (67). Although on a 129 background the animals were viable, they failed to thrive and had numerous health abnormalities, dying within the first 8 weeks of age (67). These data reveal that the removal of the single double bond on the sphingosine backbone of the sphingolipids has enormous consequences on cell function that are incompatible with healthy life.
Studies involving DES1 knockdown or utilizing embryonic fibroblasts from DES1 knock-out mice reveal the importance of this reaction in numerous cellular events, and the utilization of inhibitors of upstream components in the de novo pathway allows one to distinguish whether the remarkable phenotypic changes brought about by these interventions are due to the presence of dihydrosphingolipids or the absence of sphingolipids composed of ceramides. Nonetheless, mechanistic studies have generally failed to identify the molecular basis that allows the cell to sense the double bond or to dissect how change in membrane fluidity or integrity might contribute to the biological consequences of dihydroceramide accumulation. Filling in this gap in knowledge is essential for understanding their role in biology and represents a critical and difficult future challenge.
Collectively, the studies reviewed herein reveal an emerging area of research on a fascinating but understudied class of sphingolipids. Continued research on the biological function of these molecules could uncover novel therapeutic opportunities for targeting the DES1 enzyme. The authors of this study encourage researchers to investigate the intriguing consequences of inserting or deleting this essential double bond.
This is the second article in the Thematic Minireview series “Novel Bioactive Sphingolipids.” The authors declare that they have no conflicts of interest with the contents of this article.
Y. Li and S. A. Summers, unpublished observation.
- DES
- dihydroceramide desaturase
- ROS
- reactive oxygen species
- TLR
- Toll-like receptor.
References
- 1. Merrill A. H., Jr. (2002) De novo sphingolipid biosynthesis: a necessary, but dangerous, pathway. J. Biol. Chem. 277, 25843–25846 [DOI] [PubMed] [Google Scholar]
- 2. Omae F., Miyazaki M., Enomoto A., Suzuki M., Suzuki Y., Suzuki A. (2004) DES2 protein is responsible for phytoceramide biosynthesis in the mouse small intestine. Biochem. J. 379, 687–695 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Ternes P., Franke S., Zähringer U., Sperling P., Heinz E. (2002) Identification and characterization of a sphingolipid Δ4-desaturase family. J. Biol. Chem. 277, 25512–25518 [DOI] [PubMed] [Google Scholar]
- 4. Brockman H. L., Momsen M. M., Brown R. E., He L., Chun J., Byun H. S., Bittman R. (2004) The 4,5-double bond of ceramide regulates its dipole potential, elastic properties, and packing behavior. Biophys. J. 87, 1722–1731 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Kok J. W., Nikolova-Karakashian M., Klappe K., Alexander C., Merrill A. H., Jr. (1997) Dihydroceramide biology. Structure-specific metabolism and intracellular localization. J. Biol. Chem. 272, 21128–21136 [DOI] [PubMed] [Google Scholar]
- 6. Obeid L. M., Linardic C. M., Karolak L. A., Hannun Y. A. (1993) Programmed cell death induced by ceramide. Science 259, 1769–1771 [DOI] [PubMed] [Google Scholar]
- 7. Bielawska A., Crane H. M., Liotta D., Obeid L. M., Hannun Y. A. (1993) Selectivity of ceramide-mediated biology. Lack of activity of erythro-dihydroceramide. J. Biol. Chem. 268, 26226–26232 [PubMed] [Google Scholar]
- 8. Simon C. G., Jr., Gear A. R. (1998) Membrane-destabilizing properties of C2-ceramide may be responsible for its ability to inhibit platelet aggregation. Biochemistry 37, 2059–2069 [DOI] [PubMed] [Google Scholar]
- 9. Mitoma J., Ito M., Furuya S., Hirabayashi Y. (1998) Bipotential roles of ceramide in the growth of hippocampal neurons: promotion of cell survival and dendritic outgrowth in dose- and developmental stage-dependent manners. J. Neurosci. Res. 51, 712–722 [DOI] [PubMed] [Google Scholar]
- 10. Ueda N., Kaushal G. P., Hong X., Shah S. V. (1998) Role of enhanced ceramide generation in DNA damage and cell death in chemical hypoxic injury to LLC-PK1 cells. Kidney Int. 54, 399–406 [DOI] [PubMed] [Google Scholar]
- 11. Chik C. L., Li B., Negishi T., Karpinski E., Ho A. K. (1999) Ceramide inhibits L-type calcium channel currents in rat pinealocytes. Endocrinology 140, 5682–5690 [DOI] [PubMed] [Google Scholar]
- 12. Bao H. F., Zhang Z. R., Liang Y. Y., Ma J. J., Eaton D. C., Ma H. P. (2007) Ceramide mediates inhibition of the renal epithelial sodium channel by tumor necrosis factor-α through protein kinase C. Am. J. Physiol. Renal Physiol. 293, F1178–F1186 [DOI] [PubMed] [Google Scholar]
- 13. Summers S. A., Garza L. A., Zhou H., Birnbaum M. J. (1998) Regulation of insulin-stimulated glucose transporter GLUT4 translocation and Akt kinase activity by ceramide. Mol. Cell. Biol. 18, 5457–5464 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Zheng W., Kollmeyer J., Symolon H., Momin A., Munter E., Wang E., Kelly S., Allegood J. C., Liu Y., Peng Q., Ramaraju H., Sullards M. C., Cabot M., Merrill A. H., Jr. (2006) Ceramides and other bioactive sphingolipid backbones in health and disease: lipidomic analysis, metabolism and roles in membrane structure, dynamics, signaling and autophagy. Biochim. Biophys. Acta 1758, 1864–1884 [DOI] [PubMed] [Google Scholar]
- 15. Rahmaniyan M., Curley R. W., Jr., Obeid L. M., Hannun Y. A., Kraveka J. M. (2011) Identification of dihydroceramide desaturase as a direct in vitro target for fenretinide. J. Biol. Chem. 286, 24754–24764 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Mukhopadhyay S., Panda P. K., Sinha N., Das D. N., Bhutia S. K. (2014) Autophagy and apoptosis: where do they meet? Apoptosis 19, 555–566 [DOI] [PubMed] [Google Scholar]
- 17. Wirawan E., Vanden Berghe T., Lippens S., Agostinis P., Vandenabeele P. (2012) Autophagy: for better or for worse. Cell Res. 22, 43–61 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Shen S., Kepp O., Kroemer G. (2012) The end of autophagic cell death? Autophagy 8, 1–3 [DOI] [PubMed] [Google Scholar]
- 19. Gagliostro V., Casas J., Caretti A., Abad J. L., Tagliavacca L., Ghidoni R., Fabrias G., Signorelli P. (2012) Dihydroceramide delays cell cycle G1/S transition via activation of ER stress and induction of autophagy. Int. J. Biochem. Cell Biol. 44, 2135–2143 [DOI] [PubMed] [Google Scholar]
- 20. Signorelli P., Munoz-Olaya J. M., Gagliostro V., Casas J., Ghidoni R., Fabriàs G. (2009) Dihydroceramide intracellular increase in response to resveratrol treatment mediates autophagy in gastric cancer cells. Cancer Lett.282, 238–243 [DOI] [PubMed] [Google Scholar]
- 21. Siddique M. M., Li Y., Wang L., Ching J., Mal M., Ilkayeva O., Wu Y. J., Bay B. H., Summers S. A. (2013) Ablation of dihydroceramide desaturase 1, a therapeutic target for the treatment of metabolic diseases, simultaneously stimulates anabolic and catabolic signaling. Mol. Cell. Biol. 33, 2353–2369 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Sims K., Haynes C. A., Kelly S., Allegood J. C., Wang E., Momin A., Leipelt M., Reichart D., Glass C. K., Sullards M. C., Merrill A. H., Jr. (2010) Kdo2-lipid A, a TLR4-specific agonist, induces de novo sphingolipid biosynthesis in RAW264.7 macrophages, which is essential for induction of autophagy. J. Biol. Chem. 285, 38568–38579 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Devlin C. M., Lahm T., Hubbard W. C., Van Demark M., Wang K. C., Wu X., Bielawska A., Obeid L. M., Ivan M., Petrache I. (2011) Dihydroceramide-based response to hypoxia. J. Biol. Chem. 286, 38069–38078 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Azzam R., Hariri F., El-Hachem N., Kamar A., Dbaibo G., Nemer G., Bitar F. (2013) Regulation of de novo ceramide synthesis: the role of dihydroceramide desaturase and transcriptional factors NFATC and Hand2 in the hypoxic mouse heart. DNA Cell Biol. 32, 310–319 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Buist P. H. (2004) Fatty acid desaturases: selecting the dehydrogenation channel. Nat. Prod. Rep. 21, 249–262 [DOI] [PubMed] [Google Scholar]
- 26. Menuz V., Howell K. S., Gentina S., Epstein S., Riezman I., Fornallaz-Mulhauser M., Hengartner M. O., Gomez M., Riezman H., Martinou J. C. (2009) Protection of C. elegans from anoxia by HYL-2 ceramide synthase. Science 324, 381–384 [DOI] [PubMed] [Google Scholar]
- 27. Rodriguez-Cuenca S., Barbarroja N., Vidal-Puig A. (2015) Dihydroceramide desaturase 1, the gatekeeper of ceramide induced lipotoxicity. Biochim. Biophys. Acta 1851, 40–50 [DOI] [PubMed] [Google Scholar]
- 28. Kraveka J. M., Li L., Szulc Z. M., Bielawski J., Ogretmen B., Hannun Y. A., Obeid L. M., Bielawska A. (2007) Involvement of dihydroceramide desaturase in cell cycle progression in human neuroblastoma cells. J. Biol. Chem. 282, 16718–16728 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Fabrias G., Muñoz-Olaya J., Cingolani F., Signorelli P., Casas J., Gagliostro V., Ghidoni R. (2012) Dihydroceramide desaturase and dihydrosphingolipids: debutant players in the sphingolipid arena. Prog. Lipid Res. 51, 82–94 [DOI] [PubMed] [Google Scholar]
- 30. Nichols F. C., Housley W. J., O'Conor C. A., Manning T., Wu S., Clark R. B. (2009) Unique lipids from a common human bacterium represent a new class of Toll-like receptor 2 ligands capable of enhancing autoimmunity. Am. J. Pathol. 175, 2430–2438 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Nichols F. C., Rojanasomsith K. (2006) Porphyromonas gingivalis lipids and diseased dental tissues. Oral Microbiol. Immunol. 21, 84–92 [DOI] [PubMed] [Google Scholar]
- 32. Nichols F. C., Riep B., Mun J., Morton M. D., Bojarski M. T., Dewhirst F. E., Smith M. B. (2004) Structures and biological activity of phosphorylated dihydroceramides of Porphyromonas gingivalis. J. Lipid Res. 45, 2317–2330 [DOI] [PubMed] [Google Scholar]
- 33. Nichols F. C., Yao X., Bajrami B., Downes J., Finegold S. M., Knee E., Gallagher J. J., Housley W. J., Clark R. B. (2011) Phosphorylated dihydroceramides from common human bacteria are recovered in human tissues. PLoS One 6, e16771. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Qiang W., Sun Y., Weliky D. P. (2009) A strong correlation between fusogenicity and membrane insertion depth of the HIV fusion peptide. Proc. Natl. Acad. Sci. U.S.A. 106, 15314–15319 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Vieira C. R., Munoz-Olaya J. M., Sot J., Jiménez-Baranda S., Izquierdo-Useros N., Abad J. L., Apellániz B., Delgado R., Martinez-Picado J., Alonso A., Casas J., Nieva J. L., Fabriás G., Mañes S., Goñi F. M. (2010) Dihydrosphingomyelin impairs HIV-1 infection by rigidifying liquid-ordered membrane domains. Chem. Biol. 17, 766–775 [DOI] [PubMed] [Google Scholar]
- 36. Erdreich-Epstein A., Tran L. B., Bowman N. N., Wang H., Cabot M. C., Durden D. L., Vlckova J., Reynolds C. P., Stins M. F., Groshen S., Millard M. (2002) Ceramide signaling in fenretinide-induced endothelial cell apoptosis. J. Biol. Chem. 277, 49531–49537 [DOI] [PubMed] [Google Scholar]
- 37. Delmas D., Solary E., Latruffe N. (2011) Resveratrol, a phytochemical inducer of multiple cell death pathways: apoptosis, autophagy and mitotic catastrophe. Curr. Med. Chem. 18, 1100–1121 [DOI] [PubMed] [Google Scholar]
- 38. Hail N., Jr., Kim H. J., Lotan R. (2006) Mechanisms of fenretinide-induced apoptosis. Apoptosis 11, 1677–1694 [DOI] [PubMed] [Google Scholar]
- 39. Wu J. M., DiPietrantonio A. M., Hsieh T. C. (2001) Mechanism of fenretinide (4-HPR)-induced cell death. Apoptosis 6, 377–388 [DOI] [PubMed] [Google Scholar]
- 40. Uyama R., Hong S. H., Nakagawa T., Yazawa M., Kadosawa T., Mochizuki M., Tsujimoto H., Nishimura R., Sasaki N. (2005) Establishment and characterization of eight feline mammary adenocarcinoma cell lines. J. Vet. Med. Sci. 67, 1273–1276 [DOI] [PubMed] [Google Scholar]
- 41. Siddique M. M., Bikman B. T., Wang L., Ying L., Reinhardt E., Shui G., Wenk M. R., Summers S. A. (2012) Ablation of dihydroceramide desaturase confers resistance to etoposide-induced apoptosis in vitro. PLoS One 7, e44042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Breen P., Joseph N., Thompson K., Kraveka J. M., Gudz T. I., Li L., Rahmaniyan M., Bielawski J., Pierce J. S., Van Buren E., Bhatti G., Separovic D. (2013) Dihydroceramide desaturase knockdown impacts sphingolipids and apoptosis after photodamage in human head and neck squamous carcinoma cells. Anticancer Res. 33, 77–84 [PMC free article] [PubMed] [Google Scholar]
- 43. Ruvolo P. P., Deng X., Ito T., Carr B. K., May W. S. (1999) Ceramide induces Bcl2 dephosphorylation via a mechanism involving mitochondrial PP2A. J. Biol. Chem. 274, 20296–20300 [DOI] [PubMed] [Google Scholar]
- 44. Lee H., Rotolo J. A., Mesicek J., Penate-Medina T., Rimner A., Liao W. C., Yin X., Ragupathi G., Ehleiter D., Gulbins E., Zhai D., Reed J. C., Haimovitz-Friedman A., Fuks Z., Kolesnick R. (2011) Mitochondrial ceramide-rich macrodomains functionalize Bax upon irradiation. PLoS One 6, e19783. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Siskind L. J., Colombini M. (2000) The lipids C2- and C16-ceramide form large stable channels. Implications for apoptosis. J. Biol. Chem. 275, 38640–38644 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Siskind L. J., Kolesnick R. N., Colombini M. (2002) Ceramide channels increase the permeability of the mitochondrial outer membrane to small proteins. J. Biol. Chem. 277, 26796–26803 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Colombini M. (2013) Membrane channels formed by ceramide. Handb. Exp. Pharmacol. 109–126, 10.1007/978-3-7091-1368-4_6 [DOI] [PubMed] [Google Scholar]
- 48. Zhou H., Summers S. A., Birnbaum M. J., Pittman R. N. (1998) Inhibition of Akt kinase by cell-permeable ceramide and its implications for ceramide-induced apoptosis. J. Biol. Chem. 273, 16568–16575 [DOI] [PubMed] [Google Scholar]
- 49. Grassmé H., Jendrossek V., Bock J., Riehle A., Gulbins E. (2002) Ceramide-rich membrane rafts mediate CD40 clustering. J. Immunol. 168, 298–307 [DOI] [PubMed] [Google Scholar]
- 50. Grassmé H., Schwarz H., Gulbins E. (2001) Molecular mechanisms of ceramide-mediated CD95 clustering. Biochem. Biophys. Res. Commun. 284, 1016–1030 [DOI] [PubMed] [Google Scholar]
- 51. Grassme H., Jekle A., Riehle A., Schwarz H., Berger J., Sandhoff K., Kolesnick R., Gulbins E. (2001) CD95 signaling via ceramide-rich membrane rafts. J. Biol. Chem. 276, 20589–20596 [DOI] [PubMed] [Google Scholar]
- 52. Colombini M. (2010) Ceramide channels and their role in mitochondria-mediated apoptosis. Biochim. Biophys. Acta 1797, 1239–1244 [DOI] [PubMed] [Google Scholar]
- 53. Young M. M., Kester M., Wang H. G. (2013) Sphingolipids: regulators of crosstalk between apoptosis and autophagy. J. Lipid Res. 54, 5–19 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54. Tirodkar T. S., Voelkel-Johnson C. (2012) Sphingolipids in apoptosis. Exp. Oncol. 34, 231–242 [PubMed] [Google Scholar]
- 55. Ruvolo P. P. (2003) Intracellular signal transduction pathways activated by ceramide and its metabolites. Pharmacol. Res. 47, 383–392 [DOI] [PubMed] [Google Scholar]
- 56. van Blitterswijk W. J., van der Luit A. H., Veldman R. J., Verheij M., Borst J. (2003) Ceramide: second messenger or modulator of membrane structure and dynamics? Biochem. J. 369, 199–211 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57. Stiban J., Fistere D., Colombini M. (2006) Dihydroceramide hinders ceramide channel formation: Implications on apoptosis. Apoptosis 11, 773–780 [DOI] [PubMed] [Google Scholar]
- 58. Maurer B. J., Metelitsa L. S., Seeger R. C., Cabot M. C., Reynolds C. P. (1999) Increase of ceramide and induction of mixed apoptosis/necrosis by N-(4-hydroxyphenyl)-retinamide in neuroblastoma cell lines. J. Natl. Cancer Inst. 91, 1138–1146 [DOI] [PubMed] [Google Scholar]
- 59. Sun S. Y., Yue P., Lotan R. (1999) Induction of apoptosis by N-(4-hydroxyphenyl)retinamide and its association with reactive oxygen species, nuclear retinoic acid receptors, and apoptosis-related genes in human prostate carcinoma cells. Mol. Pharmacol. 55, 403–410 [PubMed] [Google Scholar]
- 60. Oridate N., Suzuki S., Higuchi M., Mitchell M. F., Hong W. K., Lotan R. (1997) Involvement of reactive oxygen species in N-(4-hydroxyphenyl)retinamide-induced apoptosis in cervical carcinoma cells. J. Natl. Cancer Inst. 89, 1191–1198 [DOI] [PubMed] [Google Scholar]
- 61. Apraiz A., Idkowiak-Baldys J., Nieto-Rementería N., Boyano M. D., Hannun Y. A., Asumendi A. (2012) Dihydroceramide accumulation and reactive oxygen species are distinct and nonessential events in 4-HPR-mediated leukemia cell death. Biochem. Cell Biol. 90, 209–223 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62. Idkowiak-Baldys J., Apraiz A., Li L., Rahmaniyan M., Clarke C. J., Kraveka J. M., Asumendi A., Hannun Y. A. (2010) Dihydroceramide desaturase activity is modulated by oxidative stress. Biochem. J. 427, 265–274 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63. Geeraert L., Mannaerts G. P., van Veldhoven P. P. (1997) Conversion of dihydroceramide into ceramide: involvement of a desaturase. Biochem. J. 327, 125–132 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64. Chavez J. A., Summers S. A. (2012) A ceramide-centric view of insulin resistance. Cell Metab. 15, 585–594 [DOI] [PubMed] [Google Scholar]
- 65. Bikman B. T., Summers S. A. (2011) Ceramides as modulators of cellular and whole-body metabolism. J. Clin. Invest. 121, 4222–4230 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66. Shimabukuro M., Higa M., Zhou Y. T., Wang M. Y., Newgard C. B., Unger R. H. (1998) Lipoapoptosis in beta-cells of obese prediabetic fa/fa rats: role of serine palmitoyltransferase overexpression. J. Biol. Chem. 273, 32487–32490 [DOI] [PubMed] [Google Scholar]
- 67. Holland W. L., Brozinick J. T., Wang L. P., Hawkins E. D., Sargent K. M., Liu Y., Narra K., Hoehn K. L., Knotts T. A., Siesky A., Nelson D. H., Karathanasis S. K., Fontenot G. K., Birnbaum M. J., Summers S. A. (2007) Inhibition of ceramide synthesis ameliorates glucocorticoid-, saturated-fat-, and obesity-induced insulin resistance. Cell Metab. 5, 167–179 [DOI] [PubMed] [Google Scholar]
- 68. Lopez X., Goldfine A. B., Holland W. L., Gordillo R., Scherer P. E. (2013) Plasma ceramides are elevated in female children and adolescents with type 2 diabetes. J. Pediatr. Endocrinol. Metab. 26, 995–998 [DOI] [PubMed] [Google Scholar]
- 69. Mamtani M., Meikle P. J., Kulkarni H., Weir J. M., Barlow C. K., Jowett J. B., Bellis C., Dyer T. D., Almasy L., Mahaney M. C., Duggirala R., Comuzzie A. G., Blangero J., Curran J. E. (2014) Plasma dihydroceramide species associate with waist circumference in Mexican American families. Obesity 22, 950–956 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70. Brozinick J. T., Hawkins E., Hoang Bui H., Kuo M. S., Tan B., Kievit P., Grove K. (2013) Plasma sphingolipids are biomarkers of metabolic syndrome in non-human primates maintained on a Western-style diet. Int. J. Obes. (Lond.) 37, 1064–1070, 10.1038/ijo.2012.191 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71. Yang Q., Graham T. E., Mody N., Preitner F., Peroni O. D., Zabolotny J. M., Kotani K., Quadro L., Kahn B. B. (2005) Serum retinol binding protein 4 contributes to insulin resistance in obesity and type 2 diabetes. Nature 436, 356–362 [DOI] [PubMed] [Google Scholar]
- 72. Preitner F., Mody N., Graham T. E., Peroni O. D., Kahn B. B. (2009) Long-term Fenretinide treatment prevents high-fat diet-induced obesity, insulin resistance, and hepatic steatosis. Am. J. Physiol. Endocrinol. Metab. 297, E1420–E1429 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73. Bikman B. T., Guan Y., Shui G., Siddique M. M., Holland W. L., Kim J. Y., Fabriàs G., Wenk M. R., Summers S. A. (2012) Fenretinide prevents lipid-induced insulin resistance by blocking ceramide biosynthesis. J. Biol. Chem. 287, 17426–17437 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74. Zhang Q. J., Holland W. L., Wilson L., Tanner J. M., Kearns D., Cahoon J. M., Pettey D., Losee J., Duncan B., Gale D., Kowalski C. A., Deeter N., Nichols A., Deesing M., Arrant C., Ruan T., Boehme C., McCamey D. R., Rou J., Ambal K., Narra K. K., Summers S. A., Abel E. D., Symons J. D. (2012) Ceramide mediates vascular dysfunction in diet-induced obesity by PP2A-mediated dephosphorylation of the eNOS-Akt complex. Diabetes 61, 1848–1859 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75. Hu W., Ross J., Geng T., Brice S. E., Cowart L. A. (2011) Differential regulation of dihydroceramide desaturase by palmitate versus monounsaturated fatty acids: implications for insulin resistance. J. Biol. Chem. 286, 16596–16605 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76. Holland W. L., Bikman B. T., Wang L. P., Yuguang G., Sargent K. M., Bulchand S., Knotts T. A., Shui G., Clegg D. J., Wenk M. R., Pagliassotti M. J., Scherer P. E., Summers S. A. (2011) Lipid-induced insulin resistance mediated by the proinflammatory receptor TLR4 requires saturated fatty acid-induced ceramide biosynthesis in mice. J. Clin. Invest. 121, 1858–1870 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77. Barbarroja N., Rodriguez-Cuenca S., Nygren H., Camargo A., Pirraco A., Relat J., Cuadrado I., Pellegrinelli V., Medina-Gomez G., Lopez-Pedrera C., Tinahones F. J., Symons J. D., Summers S. A., Oresic M., Vidal-Puig A. (2015) Increased dihydroceramide/ceramide ratio mediated by defective expression of degs1 impairs adipocyte differentiation and function. Diabetes 64, 1180–1192 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78. Walls S. M., Jr., Attle S. J., Brulte G. B., Walls M. L., Finley K. D., Chatfield D. A., Herr D. R., Harris G. L. (2013) Identification of sphingolipid metabolites that induce obesity via misregulation of appetite, caloric intake and fat storage in Drosophila. PLoS Genet. 9, e1003970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79. Beauchamp E., Goenaga D., Le Bloc'h J., Catheline D., Legrand P., Rioux V. (2007) Myristic acid increases the activity of dihydroceramide Δ4-desaturase 1 through its N-terminal myristoylation. Biochimie 89, 1553–1561 [DOI] [PubMed] [Google Scholar]
- 80. Ezanno H., le Bloc'h J., Beauchamp E., Lagadic-Gossmann D., Legrand P., Rioux V. (2012) Myristic acid increases dihydroceramide Δ4-desaturase 1 (DES1) activity in cultured rat hepatocytes. Lipids 47, 117–128 [DOI] [PubMed] [Google Scholar]
- 81. Brodesser S., Kolter T. (2011) Dihydroceramide desaturase inhibition by a cyclopropanated dihydroceramide analog in cultured keratinocytes. J. Lipids 2011, 724015. [DOI] [PMC free article] [PubMed] [Google Scholar]