FIGURE 3.
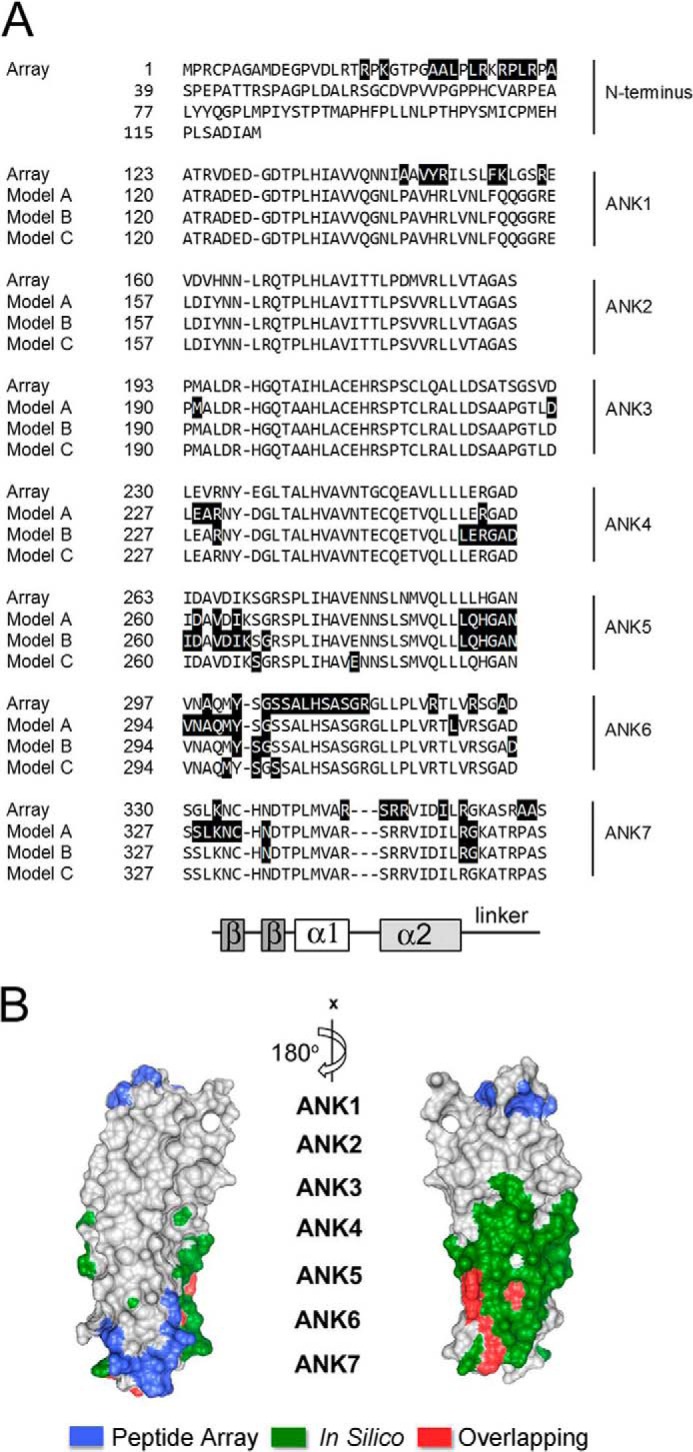
A, alignment of the seven ANK repeats of BCL-3 with a schematic indicating the conserved structural features of the each ANK repeat. Putative p50-binding residues of murine BCL-3 identified by peptide array (Array) in Fig. 2 are shaded in black and compared with p50-binding residues of human Bcl-3 predicted by the three currently available in silico models (Model A–C (15, 20)). The β hairpin (β-β), inner helix (α1), outer helix (α2), and linker regions of each ankyrin repeat are indicated. B, human BCL-3 crystal structure with corresponding unique p50-binding residues determined by peptide array (blue), p50-binding residues predicted by combined in silico Models A–C (green), and overlapping residues identified by both methods (red).
