FIGURE 8.
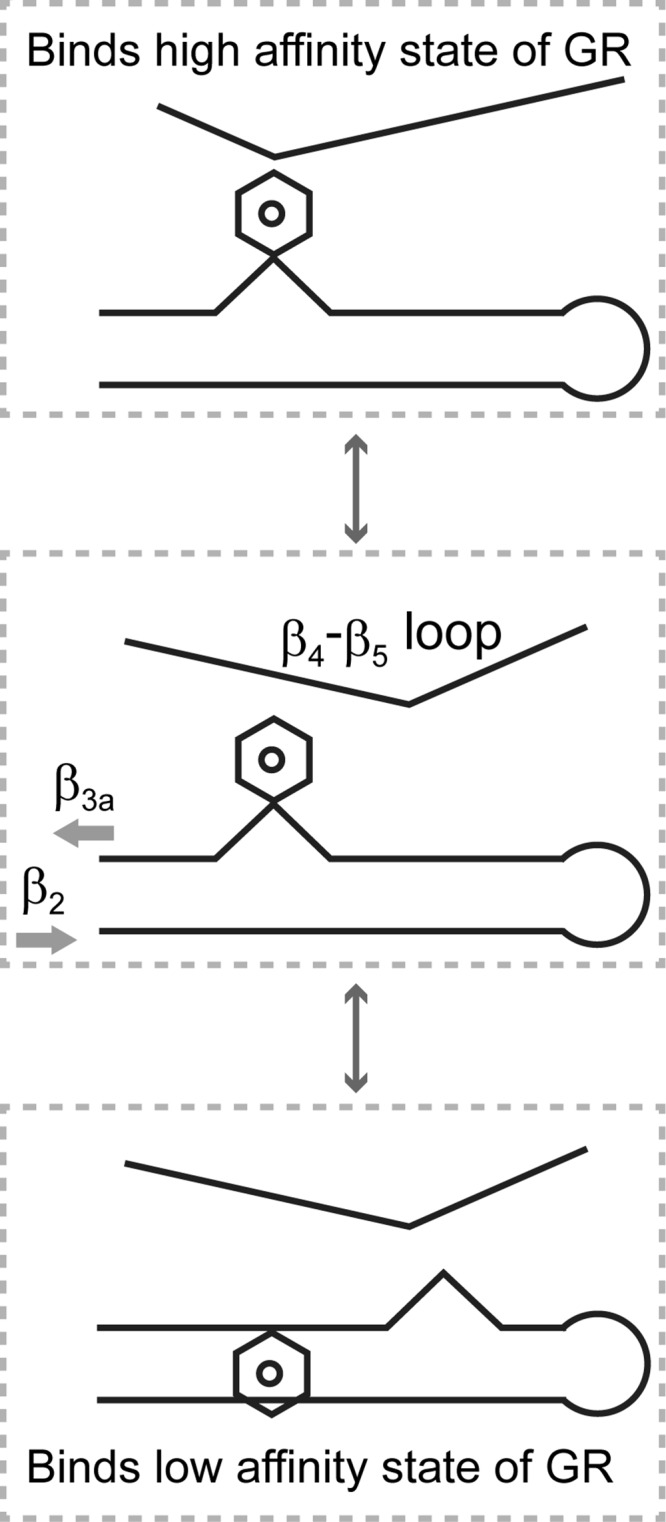
A model for the conformation-dependent modulation of glucocorticoid receptor interactions for the FK1 domains of FKBP51 and FKBP52. The β4-β5 loop and the β3a strand undergo distinct local conformational transitions in which the equilibrium population distributions appear to be energetically coupled. Transition to the alternate conformational state of the β4-β5 loop preferentially stabilizes the Phe-67-out conformation of the β3a strand in which the kink in the antiparallel hydrogen bonding pattern between the β2 and β3a strands is shifted toward the start of the β3a strand. As the transitions at both the β4-β5 loop and the β3a strand are energetically more favorable in FKBP51, the resultant conformational state is assumed to interact more strongly with the glucocorticoid receptor in its low affinity state, whereas FKBP52 primarily exists in a conformation that preferentially binds to the high affinity state of the receptor.
