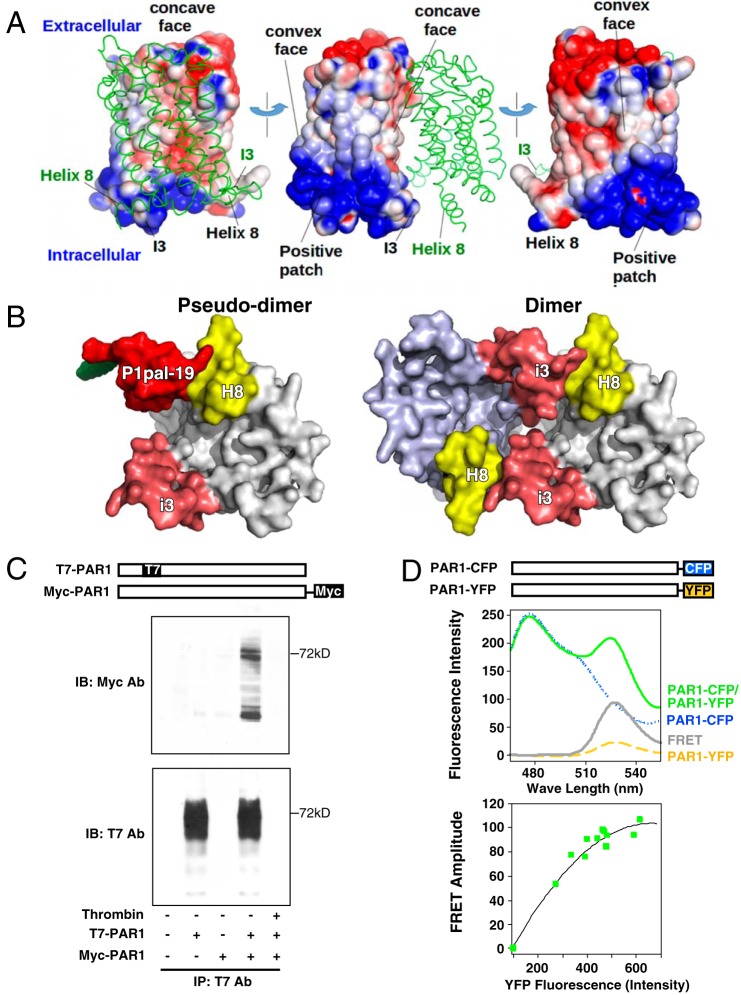
FIGURE 7.
PAR1 forms homodimers/oligomers. A, a dimer model of PAR1 was constructed by first calculating the electrostatic potential of the receptor using Advanced Poisson-Boltzmann Solver assuming 150 mm salt (62). The most intense blue and red coloring represents a potential in excess of +1 kT/e and −1 kT/e, respectively. The dimer interface was selected by minimizing electrostatic repulsions and maximizing favorable interactions. B, bottom view of proposed dimer models of PAR1 depicting the interaction of the i3 loop region of one PAR1 monomer with the H8 helix region of an adjacent PAR1 monomer using the P1pal-19/H8 helix interaction as a pseudodimer template for monomer/monomer interactions. C, co-immunoprecipitations of T7-PAR1 and PAR1-Myc were conducted in protein lysates from transfected HEK293 cells. T7-Ab-agarose beads were used to immunoprecipitate (IP) T7-PAR1 from cell lysates, and bound PAR1-Myc was detected by anti-Myc immunoblot (top). Immunoblotting (IB) with the T7-Ab (bottom) confirmed the presence of T7-PAR1. Pretreatment of HEK cells with 20 nm thrombin for 10 min prior to the collection of cell lysates resulted in complete cleavage and loss of the N-terminal T7 epitope from T7-PAR1 (and binding to beads), confirming that PAR1-Myc did not nonspecifically bind to the T7-Ab-agarose beads. D, PAR1 forms dimers in live COS7 cells. FRET between PAR1-CFP (donor) and PAR1-YFP (acceptor) was quantified in COS7 cells. Fluorescence measurements used 0.5 × 106 cells/ml with excitation at 425 nm and 10-nm slit widths. Top, yellow dashes and blue dots, respectively, represent the signal for PAR1-YFP and PAR1-CFP expressed singly. The FRET signal (gray) was determined by subtracting the background PAR1-CFP (blue) and PAR1-YFP (yellow) signals from the net uncorrected signal from co-expressed receptors (green) as described previously for PAR1-PAR4 heterodimers (22). Bottom, FRET titration between PAR1-CFP and PAR1-YFP co-expressed at different ratios in COS7 cells. The plasmid concentration of donor was kept constant, whereas the acceptor plasmid was varied. Green squares indicate the increase of the FRET amplitude as a function of fluorescence intensity of the acceptor.