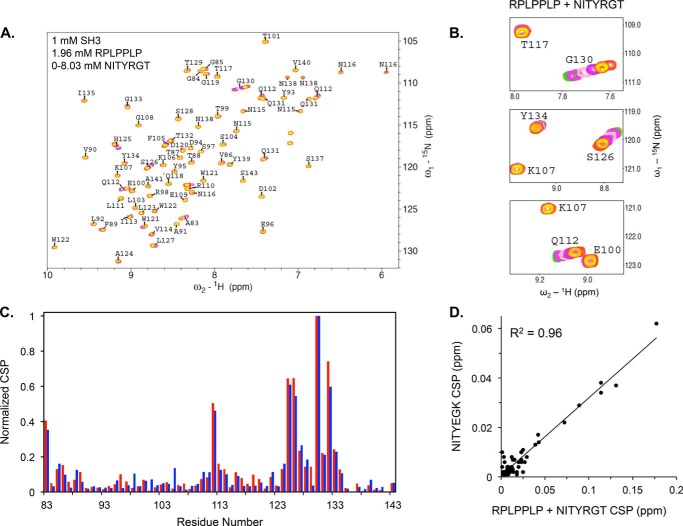
FIGURE 7.
Occupation of the PPII helix binding site in the SH3 domain by RPLPPLP prevents NITYRGT binding. A, CSP caused by NITYRGT in the HSQC spectra of the 15N-labeled c-Src SH3 domain when RPLPPLP is pre-bound to the SH3 domain. B, sub-spectra for residues Glu-100, Lys-107, Gln-112, Thr-117, Ser-126, and Gly-130 illustrating the absence of CSP in the RPLPPLP binding site and the presence of CSP in the NITYEGK interaction site. C, superimposition of normalized CSP in the c-Src SH3 domain induced by NITYEGK (blue) and by NITYRGT (red) when RPLPPLP is pre-bound to the c-Src SH3 domain. CSP were normalized by dividing the maximum CSP for each residue by the maximum CSP for Gly-130. D, correlation between the normalized CSP induced in c-Src SH3 domain by NITYEGK and by NITYRGT in the presence of RPLPPLP (R2 = 0.96).