FIGURE 8.
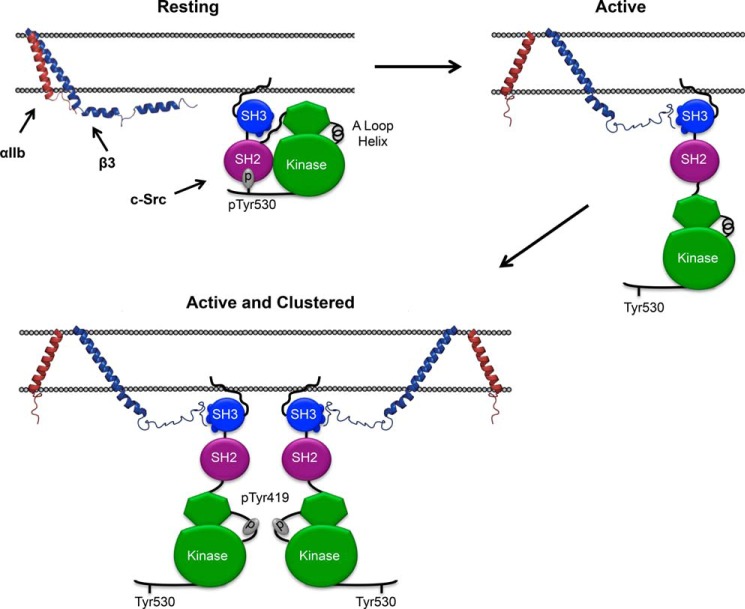
A proposed mechanism for αIIbβ3-mediated c-Src activation. In circulating platelets, where both αIIbβ3 and c-Src are present in their inactive conformations, there is no specific interaction between the β3 CT and c-Src. When platelets are stimulated, αIIbβ3 is activated, c-Src is extended, and the β3 CT is able to interact with the PPII helix binding surface of the c-Src SH3 domain that is no longer occupied by the linker between the SH2 and kinase domains. Activated αIIbβ3 then oligomerizes, enabling the trans-autophosphorylation of Tyr-419 responsible for c-Src activation.