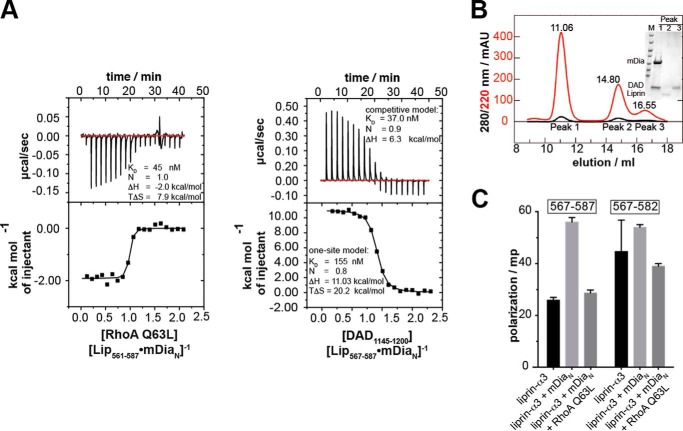
FIGURE 4.
Competition of RhoA and DAD(1145–1200) with liprin-α3 for binding toward mDiaN. A, the competition of RhoA Q63L with Lip(567–587) for mDiaN binding was studied by ITC. RhoA Q63L was titrated on a preformed complex of Lip(567–587) and mDiaN. The interaction showed a negative reaction enthalpy, ΔH, mainly driven by the favorable entropy, TΔS. The presence of liprin-α3 reduced the RhoA affinity toward mDiaN from 4 to 45 nm. B, competition of DAD(1145–1200) and Lip(561–587) for DID binding. The presence of liprin-α3 reduced the binding of DAD(1145–1200) to DID 4-fold (37 versus 153 nm). C, the ITC cell content from B analyzed by analytical gel filtration (S75 10/300). DAD(1145–1200) quantitatively displaces Lip(561–587) from DID. The SDS-PAGE (inset) shows that the high molecular weight peak at 11.06 ml contained only DID·DAD(1145–1200), whereas liprin-α3 eluted in the second peak. D, fluorescence polarization assay. The addition of 10 μm mDiaN to 100 nm F-Lip(567–587) and F-Lip(567–582) led to an increase in the polarization signal, reflecting complex formation. The addition of 15 μm active RhoA Q63L dissociates the Lip·mDiaN complexes shown by the decrease of the polarization signal to the starting level. No ternary Lip·mDiaN·RhoA complexes are formed. Values are shown as mean ± S.D. (error bars) of three replicates.