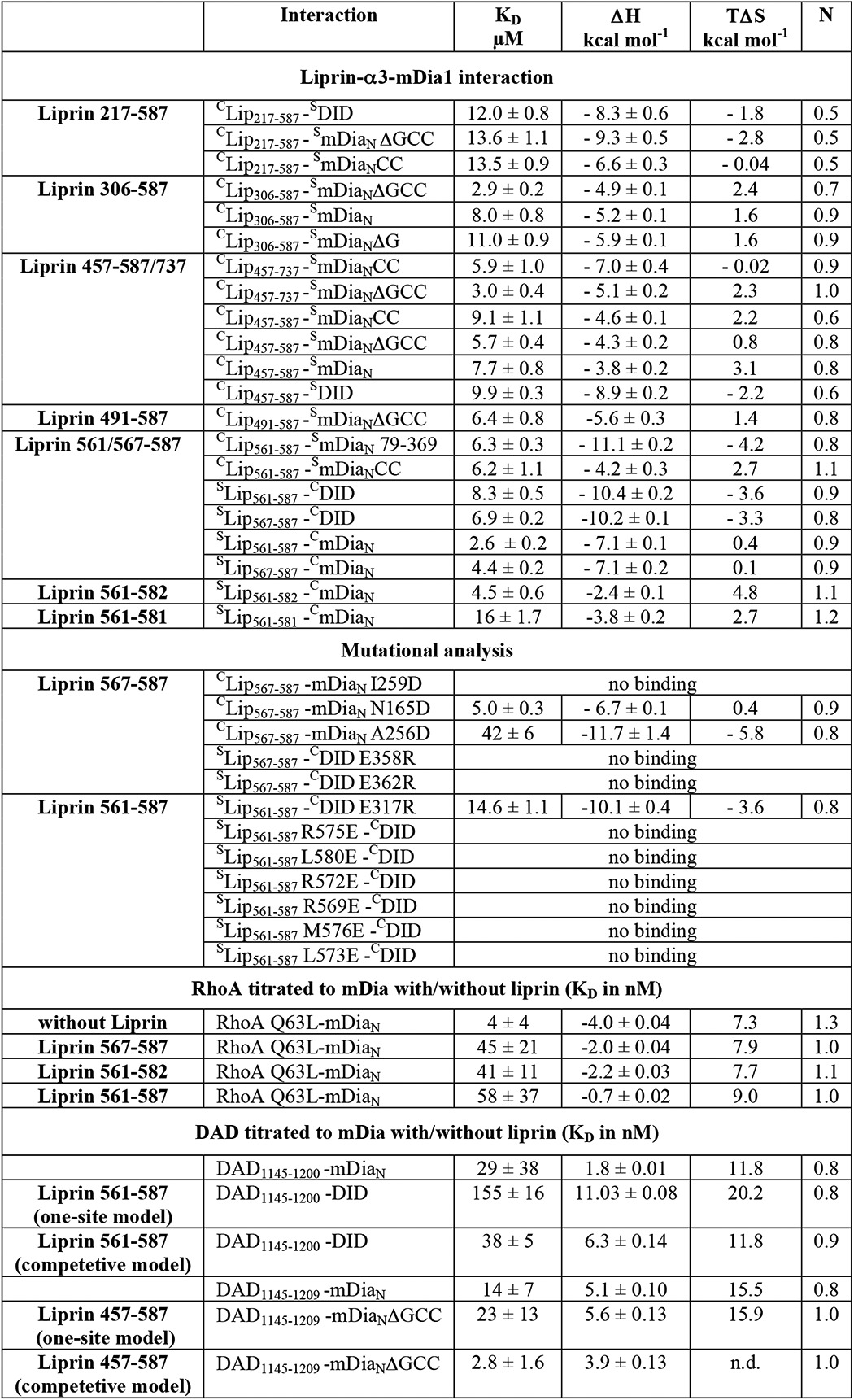
TABLE 1.
Thermodynamic characterization of liprin-α3 and mDia1 by ITC
KD is the equilibrium dissociation constant, ΔH is the enthalpy, ΔS is the entropy change, and N is the stoichiometry of the interaction. The reaction partner in the syringe (S) (300–400 μm) was titrated to the protein in the cell (C) (30–40 μm). In the cases of the competition assays, a 270 μm concentration of the protein in the cell was titrated to 27 μm complex in the cell.