FIGURE 4.
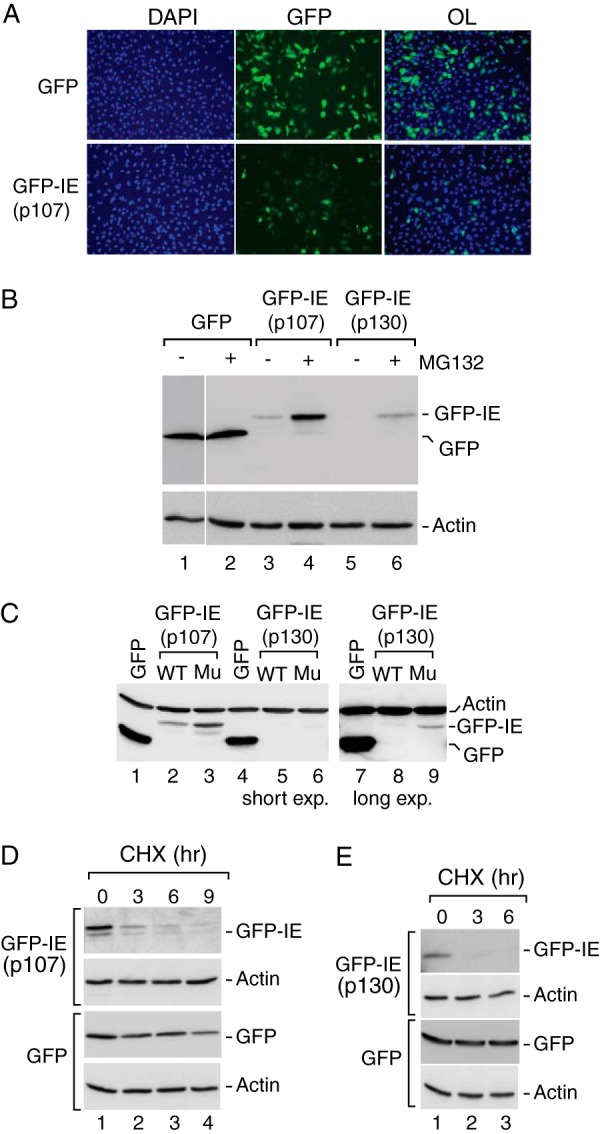
The canonical instability elements in human p107 and p130 function as autonomously acting degrons. A, the p107 IE contributes to reduced steady expression of GFP. GFP fluorescence was measured in U2OS cells transfected with GFP alone or the GFP-IEp107 chimera containing GFP fused to residues 964–1024, corresponding to the p107 instability element. Cells were counterstained with DAPI to detect cellular DNA, and overlays (OL) of the GFP and DAPI signals are shown. GFP-IEp107 showed much reduced expression as compared with GFP in most, but not all, cells. B, GFP-IEp107 and GFP-IEp130 steady state expression is enhanced by proteasome inhibition. Anti-GFP Western blot analysis was performed on whole cell extracts derived from U2OS cells transfected with GFP (lanes 1 and 2), GFP-IEp107 (lanes 3 and 4) or GFP-IEp130 containing GFP fused to residues 1035–1095 corresponding to the p130 instability element (lanes 5 and 6) in the absence or presence of MG132 as indicated. Whereas GFP remained insensitive to the proteasome inhibition, the GFP-IE fusion proteins accumulated to higher levels during MG132 treatment, suggesting the involvement of the instability element in proteasome-mediated turnover. Actin was detected as a loading control and was refractory to proteasome inhibition. C, conserved positively charged amino acids contribute to autonomous IE function. Anti-GFP Western blot analysis was performed on cells extracts transfected with either wild type GFP-IEp107 (lane 2) or mutant GFP-IEp107 containing alanine substitutions of four positively charged resides within the IE (lane 3). Similar analysis was performed for wild type GFP-IEp130 (lanes 5 and 8) and mutant GFP-IEp130 (lanes 6 and 9). In all cases the GFP chimeras containing the wild type IE sequences were expressed at lower levels than GFP alone (lanes 1, 4, and 7), whereas alanine substitution within the IE resulted in increased steady state expression. The effect of alanine substitutions on GFP-IEp130 was more evident at a higher exposure of the same blot (lane 8 versus lane 9). Actin was detected as a loading control. D and E, the p107 and p130 instability elements contribute to enhanced protein turnover. Anti-GFP Western analyses was performed on cells expressing either GFP or the wild type GFP-IE chimeras incubated in the presence of the translation inhibitor cycloheximide (CHX) for 0, 3, 6, or 9 h as indicated. GFP exhibited a half-life >9 h, whereas the half-lives of the GFP-IE chimeras were <3 h. Actin was detected as a loading control, and its levels remained unperturbed by cycloheximide treatment.
