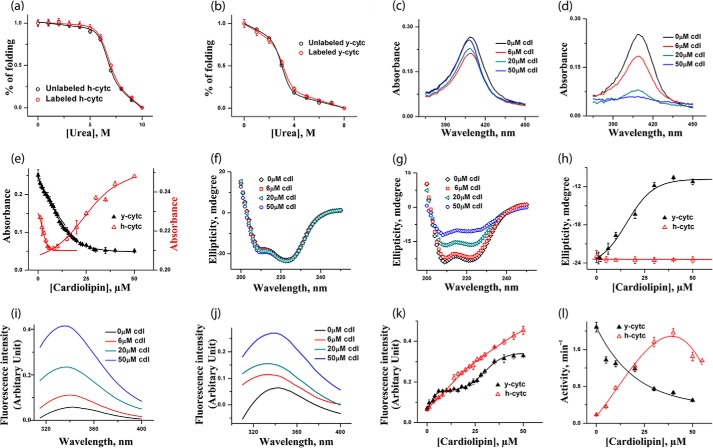
FIGURE 1.
a and b, urea-induced unfolding transitions monitored by tryptophan fluorescence of h-cyt c (a) and y-cyt c (b), where red triangles represent labeled proteins and black triangles represent unlabeled proteins. The unfolding data are fit to a two-state unfolding model, and the fits are shown by the lines. It is clear that the labeling does not change the stability of h-cyt c and y-cyt c. c and d, absorbance spectra of h-cyt c (c) and y-cyt c (d) in the presence of 0, 6, 20, and 50 μm CDL. The binding between cyt c (y-cyt c is represented in black and h-cyt c in red). CDL was monitored by the absorbance at 410 nm (e). For h-cyt c, the absorbance decreases at the first binding site, which is then followed by an increase in absorbance for the second site. For y-cyt c, in contrast, CDL binding at both sites results in a decrease in the absorbance. f and g, far-UV CD of h-cyt c (f) and y-cyt c (g) in the presence of 0, 6, 20, and 50 μm CDL. The variation of far-UV CD at 222 nm with CDL concentration for y-cyt c (black) and h-cyt c (red) is shown in h. Far-UV CD data clearly show that the binding of CDL does not lead to any significant change in the secondary structure of h-cyt c. In contrast, the secondary structure of y-cyt c decreases considerably with the increase in CDL concentrations. i and j, steady state tryptophan fluorescence data obtained with h-cyt c (i) and y-cyt c (j) in different CDL concentrations. The variation of fluorescence intensity with CDL concentrations for y-cyt c (black) and h-cyt c (red) is shown in k, and the variation in peroxidase activity with CDL concentrations is shown in l. It is interesting to note that y-cyt c has maximal activity in the absence of CDL, which decreases as the concentration of CDL increases. In contrast, the activity of h-cyt c increases with the increase in CDL concentrations, reaching a maximum at about 35 μm. All of these experiments were carried out in 20 mm phosphate buffer, pH 7.4, and at 25 °C.