FIGURE 2.
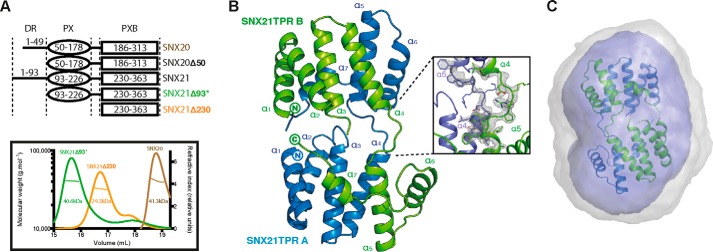
The SNX21 PXB domain crystallizes as a domain-swapped dimer. A, constructs used in this study (top) and analysis of their oligomeric state by MALLS (bottom). The center line crossing the scattering profile represents the size distribution over the peak. Asterisk indicates the presence of an N-terminal ubiquitin tag. B, top, crystal structure of the SNX21 TPR domain. The separate protein chains are colored in green and blue. The SNX21 TPR dimer reveals a domain swapping supported by the loops linking helices α4 and α5 of each chain. Refined 2Fo − Fc electron density contoured at 2.0 σ for the helices supporting the domain swapping is displayed in gray (dashed ellipse). C, the ab initio SAXS model calculated by GASBOR overlaid with the SNX21 PXB/TPR crystal structure. Averaged and filtered envelopes are shown in gray and blue, respectively. Comparison of the theoretical scattering for the SNX21 PXB/TPR domain-swapped dimer gives an excellent agreement to the experimental scattering data with a χ2 of 0.44 using CRYSOL (Table 2).
