FIGURE 9.
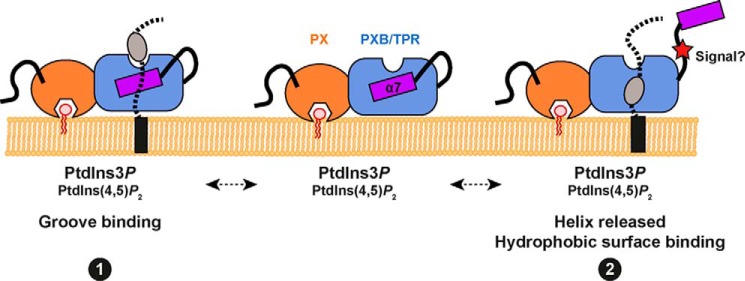
A model for membrane and ligand engagement by the SNX-PXB proteins. We propose two alternatives for coincident lipid and protein binding to the SNX-PXB proteins. The PX domain can bind the endosomal lipid PtdIns3P, as well as the lipid PtdIns(4,5)P2 although the functional significance of this is still unclear. In conjunction with membrane targeting the TPR domain of the SNX-PXB proteins may then engage ligands through an unusual binding groove similarly to the binding of the Caf4p adaptor to Fis1p (see Fig. 4C) (binding mode 1). Alternatively the atypical α7 helix could play a regulatory role, whereby ligand binding or perhaps signal-induced conformational changes such as phosphorylation of the α6-α7 linker region cause a movement in α7 allowing ligand association with the conserved hydrophobic pocket buried beneath (binding mode 2).
