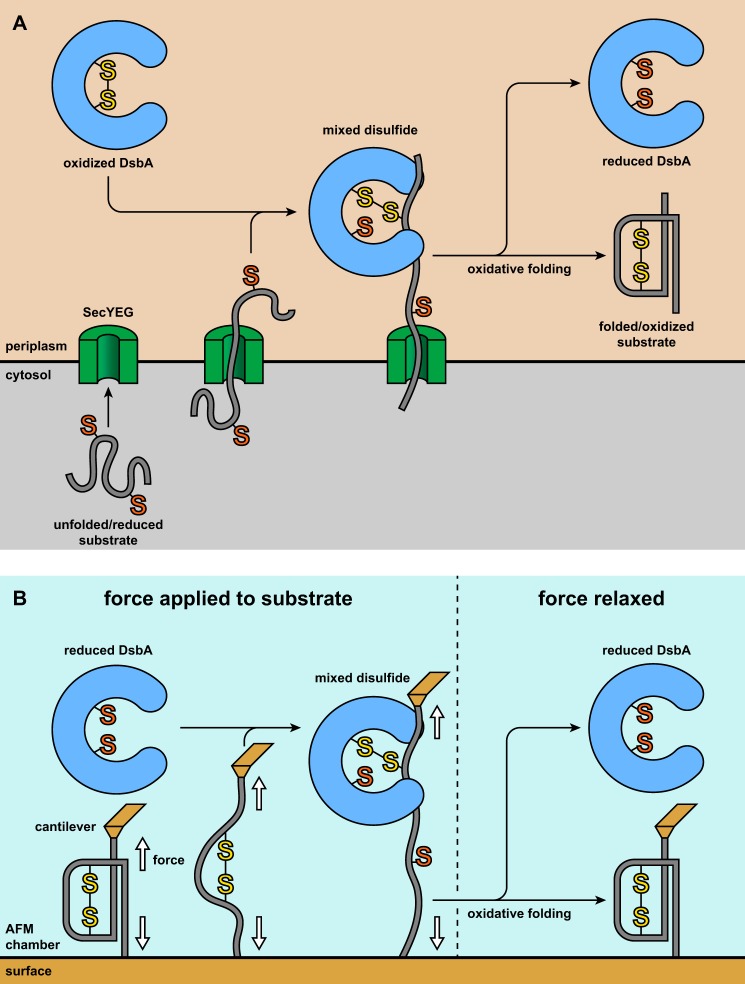
FIGURE 1.
A, in vivo, nascent proteins are translocated into the oxidative environment of the periplasm via the SecYEG translocon, emerging as a semiextended, unfolded, and reduced polypeptide. Oxidized DsbA forms a mixed disulfide with a substrate cysteine. Upon completion of translocation, the collapsed mixed disulfide complex undergoes folding and resolution of the mixed disulfide. This leads to the formation of a folded and oxidized substrate while a reduced DsbA is released. The requisite SecA ATPase, which drives the substrate through the SecYEG channel, has been omitted for clarity. B, in our experiments, we apply force to a single substrate molecule with buried disulfide bonds. Although a monomer is depicted for simplicity, the construct used in our experiments is a polyprotein consisting of eight tandem repeats of the substrate domain. Force induces unfolding whereupon the disulfide is solvent-exposed and can then be attacked by reduced DsbA, yielding a mixed disulfide complex. The redox states of the enzyme and substrate are inverted with respect to the in vivo conditions to provide a signal for mixed disulfide formation. However, once the mixed disulfide is formed, the conditions are equivalent to those of the process in vivo. We relax the force, allowing the substrate to collapse. This enables folding and oxidation, and the initial redox states of enzyme and substrate are recovered.