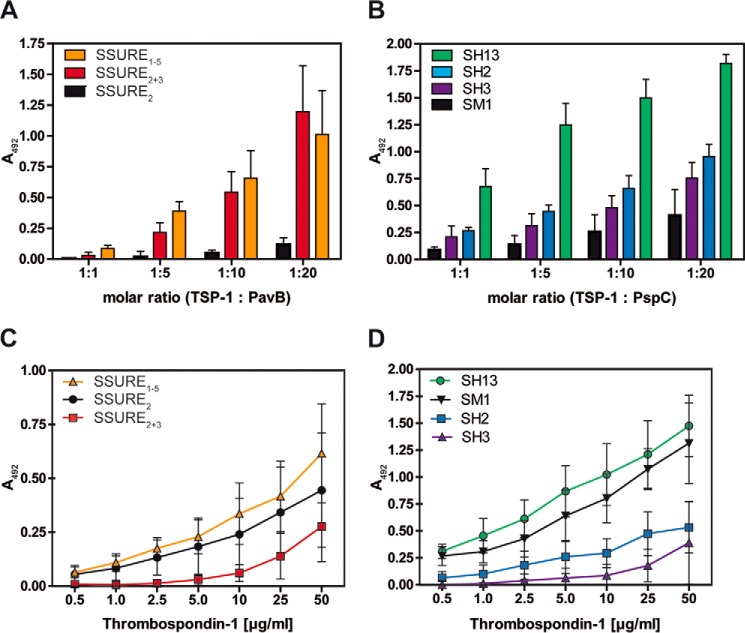
FIGURE 3.
Repetitive structures of PavB and PspC are involved in the interaction with hTSP-1. A and B, dose-dependent binding of soluble pneumococcal proteins PavB (A) or PspC (B) to immobilized hTSP-1. Human TSP-1 (0.1 μg in 100 μl/well) was coated on microtiter plates (MaxisorpTM, Nunc) and, after blocking, incubated with increasing molecular ratios of heterologously expressed PavB or PspC fragments. Bound pneumococcal proteins were detected using a polyclonal mouse anti-SSURE2+3 (PavB) antibody or a polyclonal mouse anti-SH2 (PspC) antibody and a peroxidase-coupled secondary anti-mouse antibody. Results are illustrated as mean values ± S.D. of at least three independent experiments. C and D, concentration-dependent binding of soluble hTSP-1 to immobilized, heterologously expressed PavB and PspC proteins. Pneumococcal proteins were immobilized on microtiter plates (PolysorpTM, Nunc) in equimolar amounts related to SSURE2 or SH3 (each 0.5 μg in 50 μl/well). Binding of hTSP-1 was detected after incubating the immobilized proteins with increasing concentrations of hTSP-1 (0–50 μg/ml) using a specific polyclonal mouse anti-hTSP-1 antibody and a peroxidase-coupled secondary anti-mouse antibody. Results are illustrated as mean values ± S.D. of at least four independent experiments.