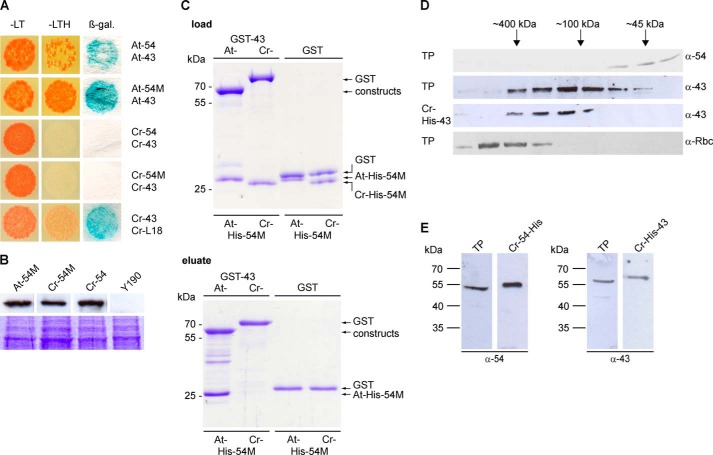
FIGURE 2.
Unlike higher plants, Chlamydomonas cpSRP54 and cpSRP43 do not form a stable complex in vitro and in vivo. A, for yeast two-hybrid assays, the yeast strain Y190 was cotransformed with pGBKT7 constructs encoding the full-length cpSRP54 (54) or its M-domain (54M) of Arabidopsis (At) and Chlamydomonas (Cr) and pACT2 constructs encoding cpSRP43 (43) of these organisms. Cotransformed cells were dotted onto minimal media lacking Leu and Trp (−LT) to check for cotransformation or −LTH to assess the extents of interaction. β-Galactosidase (β-gal.) activity was visualized using filter assays. B, expression of the pGBKT7 encoded fusion proteins in the yeast cells was verified by Western blot analysis of total yeast protein extracts (equivalent to 1 ml of a culture with an A600 of 1) using antibodies against the c-Myc epitope (BD Biosciences). Untransformed yeast cells were used as negative controls. Equal loading was controlled by a duplicate gel stained with Coomassie Blue. C, in vitro pulldown assays were performed with recombinant GST-cpSRP43 (At- and Cr-GST-43) proteins and the His-tagged M-domain of cpSRP54 proteins (At- and Cr-His-54M) as indicated using glutathione-Sepharose. Control reactions were performed with recombinant GST. One-tenth of the loaded and one-third of the eluted proteins (upper and lower panels) were separated by SDS-PAGE and detected by Coomassie staining. D, either total soluble protein extracts (TP) of Chlamydomonas or recombinant Cr-His-cpSRP43 (Cr-43) was fractionated by size exclusion chromatography, and the levels of Cr-cpSRP54, Cr-cpSRP43, and the large subunit of Rubisco (Rbc) were evaluated by Western blot analyses. E, Western blot analysis of 30 μg of a total soluble protein extract (TP) from Chlamydomonas and recombinant Cr-His-cpSRP43 (Cr-43) or Cr-cpSRP54-His (Cr-54) using antibodies directed against Cr-cpSRP54M (generated against recombinant Cr-cpSRP54M; Seqlab) or At-cpSRP43 (7).