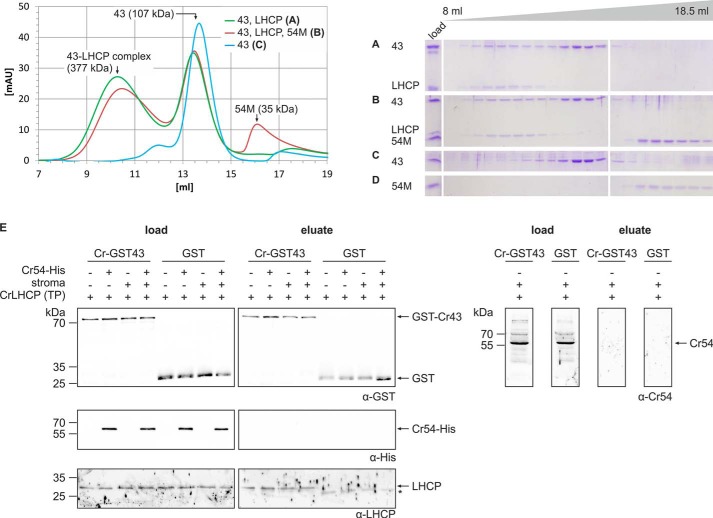
FIGURE 6.
Cr-cpSRP54 is not involved in transit complex formation in Chlamydomonas. A–D, protein complex formation using the following equimolar combinations of recombinant proteins was analyzed by size exclusion chromatography: Cr-His-cpSRP43 and Cr-LHCP (A) and Cr-His-cpSRP43 (43), Cr-LHCP (LHCP), and Cr-His-cpSRP54M (54M) (B). As controls, the single proteins Cr-His-cpSRP43 (C) and Cr-His-cpSRP54M (D) were analyzed. Elution fractions ranging from 8 to 18.5 ml were analyzed by SDS-PAGE and Coomassie staining (A–D). Cr-His-cpSRP54M was added to the combination of Cr-His-cpSRP43 and Cr-LHCP after dilution and removal of SDS (B). The same elution profiles were obtained when Cr-His-cpSRP54M was added to this combination before SDS removal.3 Single proteins were always treated in the same way as in assays analyzing complex formation. E, pulldown assays were conducted by incubation of recombinant Cr-GST-cpSRP43 and the Cr-LHCP in vitro translation product (TP) with the indicated combinations of recombinant Cr-cpSRP54-His and a stromal extract from Chlamydomonas. Control reactions were performed with recombinant GST. Proteins were enriched using glutathione-Sepharose. Samples of the load and the eluted fractions were analyzed by Western blotting using antibodies directed against the GST tag, His tag, or LHCP. The asterisk (*) marks signals caused by an unspecific cross-reaction of the α-Cr-LHCP antibody with the eluted GST protein. To detect endogenous stromal Cr-cpSRP54 (Cr-54) in the load and eluted fractions, the samples from pulldown assays conducted in the presence of Cr-GST-cpSRP43, Cr-LHCP, and stromal extract were blotted and analyzed using an antibody directed against Cr-cpSRP54M. mAU, milliabsorbance units.