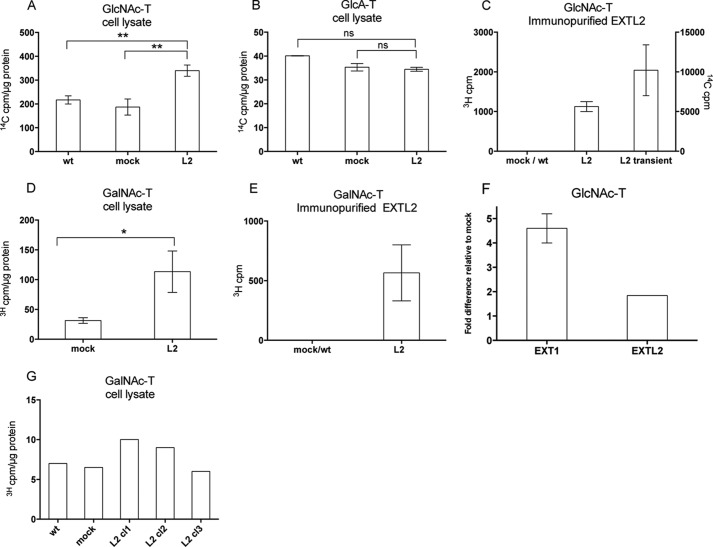
FIGURE 7.
Glycosyltransferase activities of HEK293 cells overexpressing EXTL2. Glycosyltransferase activities of cell extracts or immunopurified EXTL2, from untransfected (wt), empty vector-transfected (mock), EXTL2-transfected (L2), and EXT2-transfected HEK293 cells are shown as indicated. In A–F, oligosaccharides derived from Escherichia coli K5 capsular polysaccharide were used as acceptor substrates in transferase assays. The K5 polysaccharide has the same structure as the nonsulfated HS precursor molecule, and oligosaccharide derivatives thereof, with non-reducing terminal GlcA or GlcNAc residues served as acceptors in the GlcNAc- and GlcA-transferase reactions. C, shows the GlcNAc-transferase activity of immunopurified EXTL2 after transient transfection, whereas the other panels show enzyme activities after stable transfection. No GlcA-transferase activities were detected with immunopurified EXTL2 (data not shown). In G, oligosaccharides with a non-reducing GlcA residue, generated from defructosylated E. coli K4 capsular polysaccharide with the same structure as the nonsulfated chondroitin sulfate precursor molecule, were used as acceptor substrate in transferase assays. The error bars represent mean values ± S.D. from four (A) and two (B, D, and E) independent EXTL2-overexpressing clones and from one clone (F). G, representative results from one out of two independent analyses. Each clone was assayed at two different protein concentrations. *, p < 0.01, **, p < 0.001. ns, not significant.