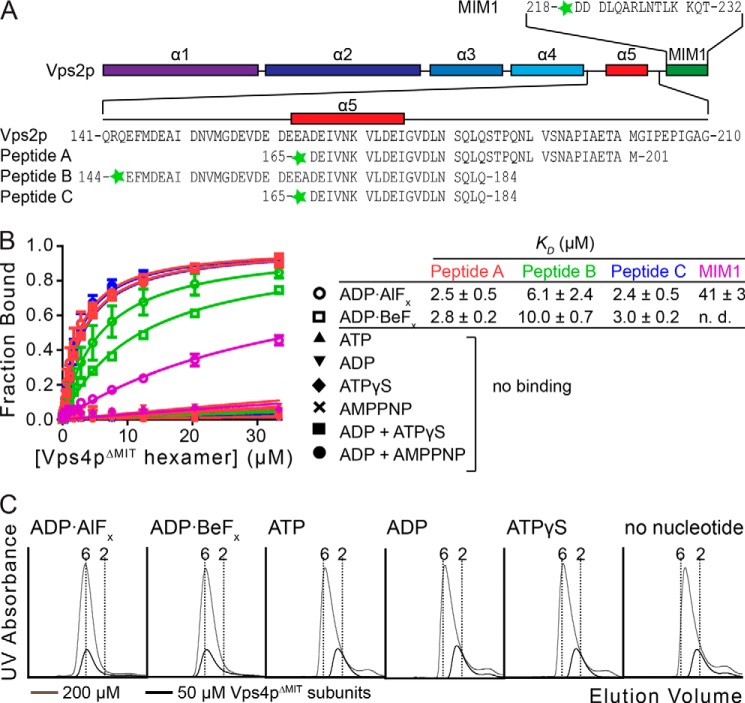
FIGURE 1.
Vps2p helix 5 peptides bind the Vps4p ATPase cassette. A, the Vps2p MIM1 and A, B, and C peptides used in this study are indicated. The predicted secondary structure of Vps2p is shown as a schematic. B, binding between peptides and Vps4p was assayed by fluorescence anisotropy. Peptides A, B, and C bind Vps4pΔMIT in the presence of ADP·AlFx or ADP·BeFx, but not in the presence of other nucleotides tested. The control MIM1 peptide also binds, but ∼15-fold more weakly. KD values and S.D. value are shown to the right. C, Vps4pΔMIT forms a stable hexamer in the presence of Vta1pVSL and ADP·AlFx or ADP·BeFx. Vps4pΔMIT at 200 μm subunit concentrations (33 μm hexamer, gray) and 50 μm subunit concentrations (8.3 μm hexamer, black) was run on a gel filtration column in the presence of Vta1pVSL and different nucleotides. Vertical dotted lines indicate calculated elution volumes of the Vps4pΔMIT hexamer and dimer relative to standards. Error bars indicate S.D. from three independent experiments.