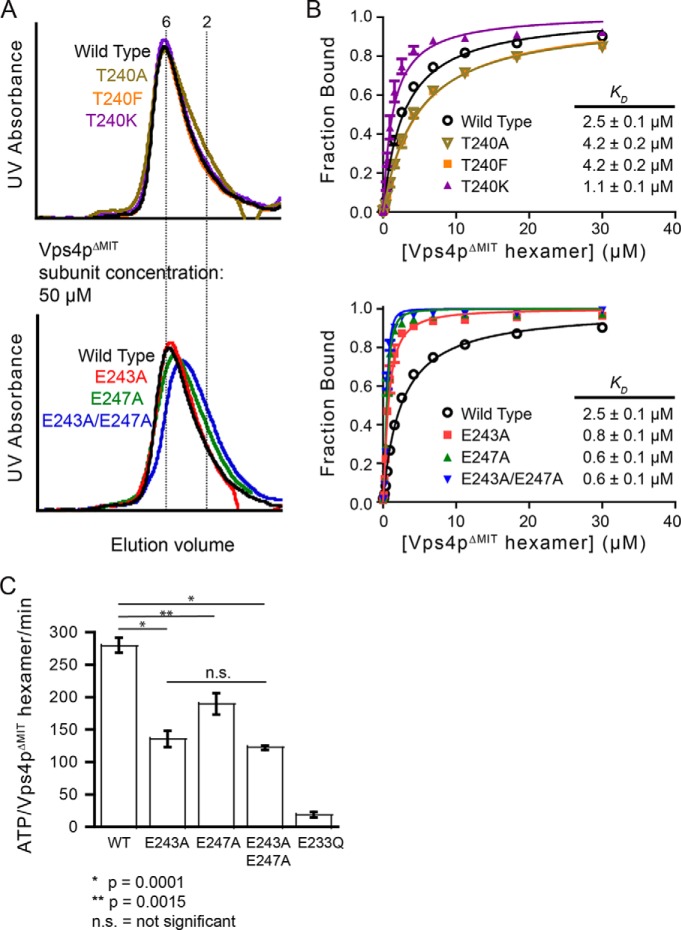
FIGURE 4.
Negatively charged residues on pore loop 2 of the Vps4p hexamer modulate binding to Vps2p helix5. A, Vps4pΔMIT carrying pore loop 2 mutations T240A, T240K, T240F, E243A, or E247A can form hexamers as assayed by gel filtration chromatography (50 μm Vps4p subunits, 8.3 μm hexamers) in the presence of Vta1pVSL and ADP·AlFx. B, Vps4pΔMIT proteins carrying mutations on Thr-240 (upper panel) or mutations of acidic pore loop 2 residues (lower panel) were tested for binding to peptide C by fluorescence anisotropy. When compared with Vps4pΔMIT, Vps4pΔMIT(T240K) and Vps4pΔMIT(E243A,E247A) displayed increased binding. C, mutations of the acidic residues on pore loop 2 of Vps4p impaired the ATPase activity of Vps4p. A double mutation (E243A,E247A) on the negatively charged collar reduced the ATPase activity by ∼55% when compared with wild-type Vps4p. The ATPase inactive mutant E233Q was used as a negative control. Error bars indicate S.D. from three independent experiments.