FIGURE 3.
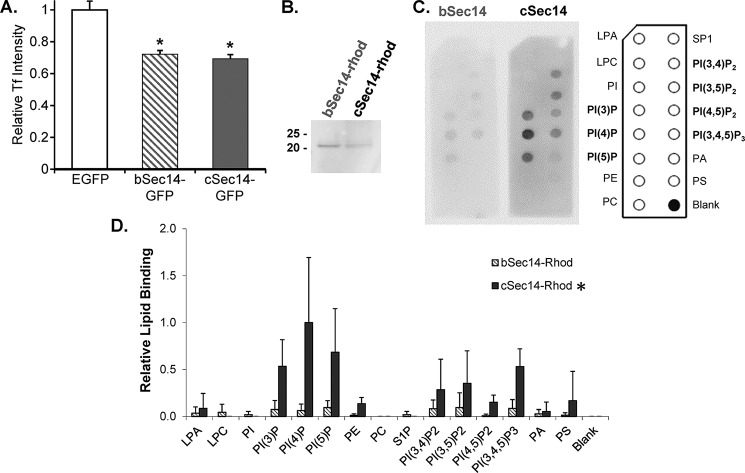
The bSec14 and cSec14 domains of kalirin both inhibit transferrin uptake but exhibit differential binding to PIP strips. A, AtT-20 cells transiently expressing EGFP, bSec14-GFP, or cSec14-GFP were rinsed in serum-free medium, exposed to fluorescently tagged transferrin for 5 min, and then rinsed and fixed. The intensity of the transferrin signal in cells expressing EGFP, bSec14-GFP, or cSec14-GFP was quantified (n = 25, 51, and 49 cells, respectively). Two-way ANOVA statistical analysis was used; *, p < 0.005 versus EGFP. B, Coomassie-stained membrane showing purified bSec14-rhod and cSec14-rhod. C, the binding of bSec14-rhod and cSec14-rhod to PIP strips was quantified using an antibody to the epitope tag. The lipids on the PIP strips are identified in the schematic to the right. D, group data from 2–4 experiments calculated as spot intensity relative to cSec14-rhod binding to PI(4)P, the preferred interactor; error bars, S.D. cSec14 binding to PIPs was significantly higher than bSec14 binding; *, p < 0.02 by two-way ANOVA.