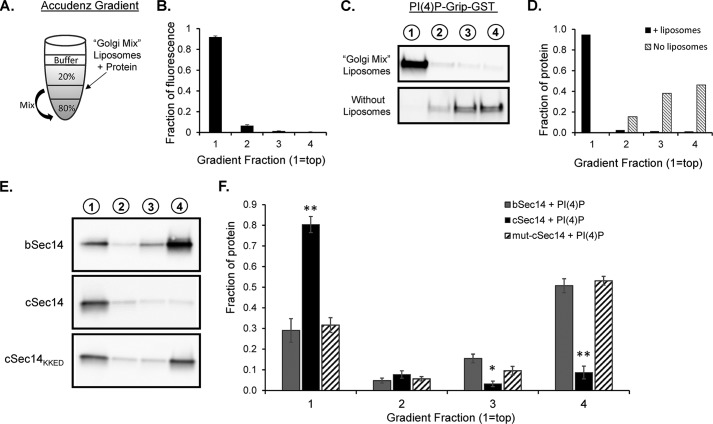
FIGURE 6.
Liposome binding assays reveal an important role for the amphipathic C-helix in KalSec14 membrane interactions. A, schematic illustrating Accudenz gradients used for the liposome flotation assays conducted in Figs. 6 and 7. The indicated liposome/protein mix (50 μl) was combined with 50 μl of 80% Accudenz and then overlayered with 250 μl of 20% Accudenz and 50 μl of buffer. Gradients were centrifuged in a swinging bucket rotor for 30 min, and 100-μl fractions were removed and analyzed for lipid and protein content. B, relative lipid fluorescence in gradient fractions; spectrophotometry data from 21 separate gradients were averaged, showing that >90% of the lipid was found in the top fraction of the gradient after centrifugation. C, purified PI(4)P-Grip-GST (positive control) was analyzed on gradients in the presence (top) or absence (bottom) of Golgi mix liposomes; gradient fractions are indicated above each lane. Protein content, detected using a GST antibody, was quantified as shown in D. E, representative blots from liposome assays with KalSec14 variants. cSec14 bound significantly more to Golgi mix liposomes than bSec14, and this difference was eliminated when the C-helix was mutated to disrupt its amphipathic nature; group data are shown in F. Error bars, S.E.; n = 4–6 experiments/protein. Asterisks indicate significant difference from bSec14 + PI(4)P liposomes by two-way ANOVA; **, p < 0.001; *, p < 0.02.