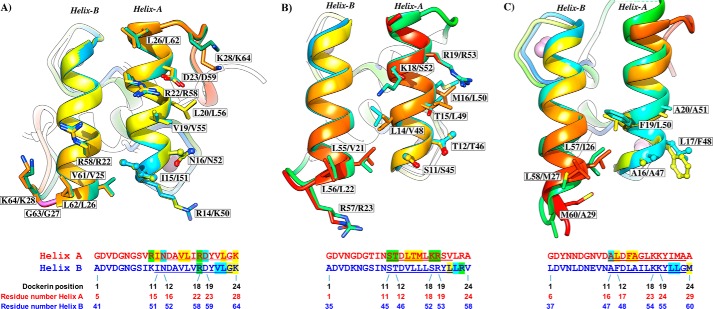
FIGURE 4.
Two identical cohesin-binding faces support the dual binding mode of type I dockerins from A. cellulolyticus (A), C. thermocellum (B), and C. cellulolyticum (C). Dockerins are color-ramped and were overlaid with its 180° rotated alternative binding mode structure. The most important cohesin contact residues are highlighted and displayed as ball-and-stick or sticks. Below the representation of the two cohesin-interacting faces, the primary sequence alignment of the respective dockerin is provided (dockerin position number and residue number indicated below each alignment). Residues in green are involved in polar contacts, yellow in hydrophobic contacts, and blue in both. Helix-A defines the helix that dominates cohesin recognition. Helix-B only contacts the cohesin at one of its termini (see text for details).