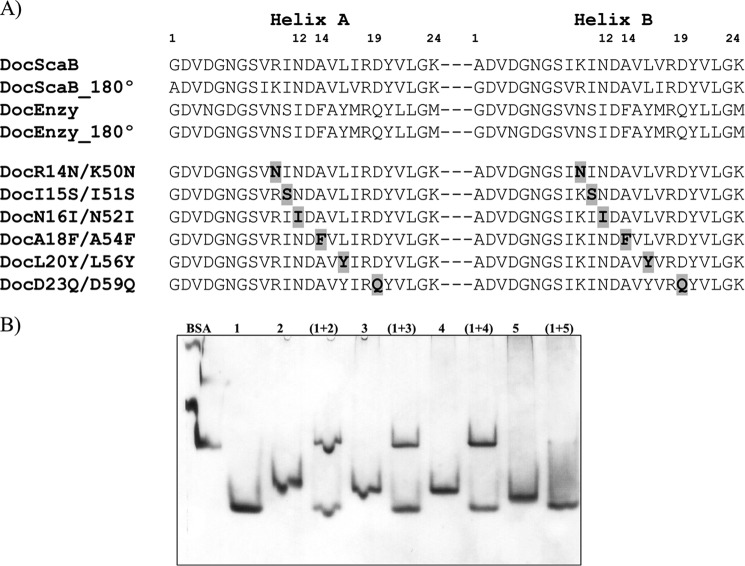
FIGURE 6.
Residue determinants of type I cohesin-dockerin specificity in A. cellulolyticus. A, identifies the residues that may modulate type I Coh-Doc specificity in A. cellulolyticus (DocScaB) aligned with the primary consensus sequence of a type I enzyme dockerin (Doc_Enzy) of A. cellulolyticus. Key residues located in either helix-A or helix-B, are highlighted in bold, within these sequences. The two cohesin-binding faces were revealed by rotating each dockerin by 180°, demonstrating the highly symmetrical nature of dockerin sequences. The importance of the proposed residues of type I specificity have been probed by producing DocScaB mutant derivatives (DocR14N/K50N to DocD23Q/D59Q) with single amino acid changes at both helices. The changes (highlighted in bold and shaded gray) were made to reflect those of the type I enzyme dockerin (Doc_Enzy). B, shows an example of the affinity of the engineered dockerins DocR14N/K50N (lane 2), DocI15S/I51S (lane 3), DocN16I/N52I (lane 4), and the type I enzyme dockerin, DocGH5, as a negative control (lane 5) for CohScaC (lane 1), evaluated qualitatively by NGE.