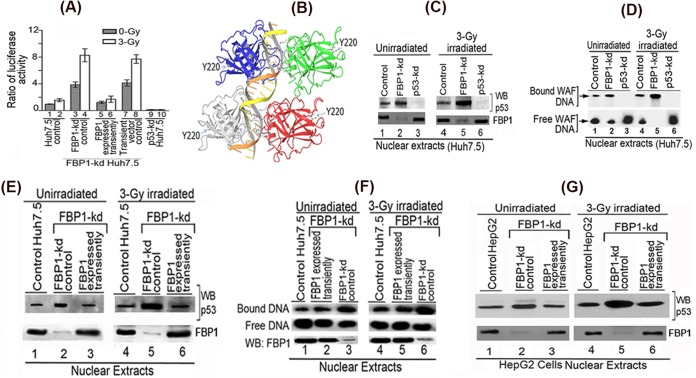
FIG 5.
Transactivation activity and DNA binding ability of mutant p53Y220C in Huh7.5 cells is activated by knockdown of FBP1 expression. (A) Transcription activity of mutant p53Y220C in control and FBP1-kd Huh7.5 cells under radiation-induced stress. The p21-luc reporter and pRL-SV40 plasmids were cotransfected into control, FBP1-kd, and FBP1-kd cells in which FBP1 was transiently expressed via transfection of an shRNA-resistant FBP1-expressing clone (pCIA-cmv-FBP1SHR). Forty-eight hours later, cells were irradiated with a 3-Gy dose of gamma irradiation. Luciferase activity was measured after 6 h postirradiation. Experiments were done in triplicate; results are expressed as the ratio of firefly luciferase to Renilla luciferase activities in cell lysate. Lanes 1 and 2, control cells; lanes 3 and 4, FBP1-kd cells; lanes 5 and 6, FBP1 transiently expressed in FBP1-kd cells; lanes 7 and 8, FBP1-kd cells transfected with vector alone; lanes 9 and 10, p53 kd cells. (B) The three-dimensional structure of homotetrameric p53-DNA binary complex and position of Y220. Using Maestro molecular modeling software, version 9.3.5 (Schrodinger, Inc.), we downloaded the backbone structure of the p53-DNA binary complex from PDB entry 4HJE (80). The three-dimensional crystal structure of DNA-bound p53 is displayed without any modification. The backbone of duplex DNA bound to the tetrameric p53 and the position of Y220 located far away from the bound DNA are shown. (C) Streptavidin magnetic bead DNA binding assay indicates enhanced DNA binding activity of mutant p53Y220C in the nuclear extract from FBP1-kd Huh7.5 cells. Normalized nuclear extracts from unirradiated or 3-Gy-γ-irradiated control and FBP1-kd cells were incubated with 30 bp biotinylated WAF-side DNA at 37°C. The nuclear extract from p53-kd cells was included as a negative control. DNA-protein complexes were captured on streptavidin paramagnetic beads, resolved on SDS-PAGE, and Western blotted for p53. The normalized nuclear extract also was Western blotted for FBP1. Lanes 1 to 3, unirradiated; lanes 4 to 6, γ-irradiated. (D) EMSA showing enhanced binding of 30 bp WAF-side DNA by p53Y220C in the nuclear extract from FBP1-kd Huh7.5 cells. Normalized nuclear extracts from unirradiated and 3-Gy-γ-irradiated control and FBP1-kd cells were incubated with 32P-labeled WAF-side DNA and then subjected to EMSA on 4% native polyacrylamide gel. p53-kd Huh7.5 cells were included as a negative control. Lanes 1 to 3, unirradiated; lanes 4 to 6, γ-irradiated. (E and F) Transient expression of FBP1 in FBP1-kd cells strongly inhibits p53Y220C binding to its target DNA. DNA binding activity of mutant p53 in normalized nuclear extract from unirradiated and irradiated control, FBP1-kd, and transiently FBP1 expressing FBP1-kd Huh7.5 cells was examined by streptavidin magnetic bead DNA binding assay (E) and EMSA (F). The normalized nuclear extract also was Western blotted for FBP1. Lanes 1 to 3, unirradiated; lanes 4 to 6, irradiated. (G) Transient expression of FBP1 in FBP1-kd HepG2 cells inhibits binding of wild-type p53 to its target DNA. DNA binding activity of wild-type p53 in a normalized nuclear extract from unirradiated and irradiated control, FBP1-kd, and transiently FBP1-expressing FBP1-kd HepG2 cells was examined by streptavidin magnetic bead DNA binding assay. The p53 bound to the target DNA was captured on streptavidin magnetic beads and Western blotted for p53. The normalized nuclear extract also was Western blotted for FBP1. Lanes 1 to 3 represent unirradiated control, FBP-kd, and FBP-kd cells, respectively, transiently expressing FBP1; lanes 4 to 6 represent irradiated control, FBP-kd, and FBP-kd cells, respectively, transiently expressing FBP1.