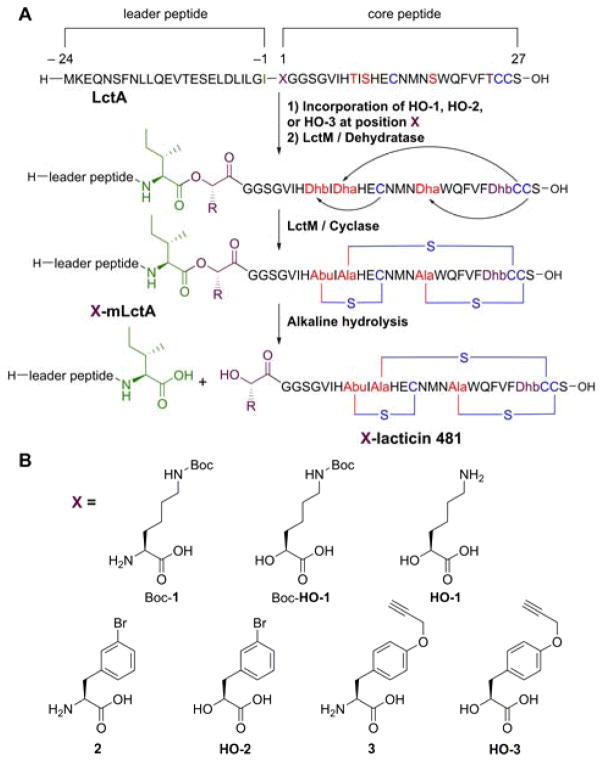
Figure 1.
A) Biosynthesis of lacticin 481 analogs by incorporating Boc-HO-1, HO-2, or HO-3 at position 1 of the core peptide (X) using the amber stop codon suppression method. WT lacticin 481 contains a lysine at position 1. Also, position –1 (green) was mutated from Ala to Ile as described in the text. B) Structures of non-proteinogenic amino and hydroxy acids introduced into lacticin 481 or nukacin ISK-1 at residue 1 (X).