Figure 5.
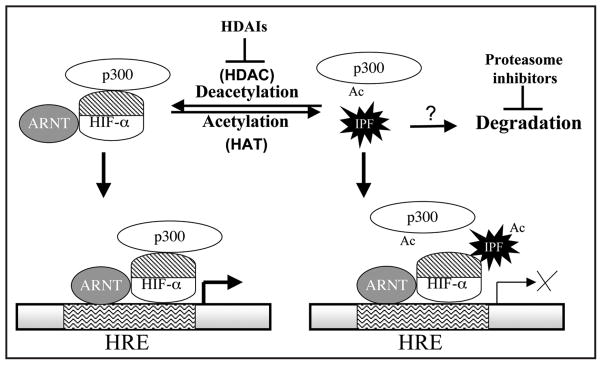
Possible molecular basis underlying the HDAI-mediated repression of HIF-αCAD TAP. A deacetylation event is proposed to be essential for the function of HIF-αCAD/p300 complex. The addition of HDAIs blocks the deacetylation event and causes the hyperacetylation of an inhibitory protein factor (IPF) or p300. The eventual consequence would be a change in the formation of HIF-αCAD/p300 complex. Proteasome inhibitors may enhance the levels of IPF, thus repressing HIF function in a similar model. ARNT: aryl hydrocarbon receptor nuclear translocator, the dimerization partner of HIF-α, also known as HIF-1β.
