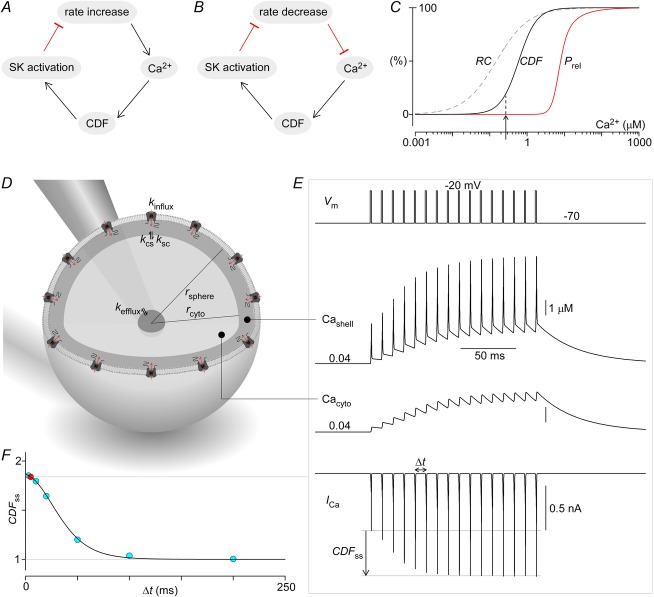
Figure 11. Neurophysiological implications of CaV2.1 CDF.
A, diagram illustrating the role of CDF in homeostatic rate control of Purkinje cells. Increased rate of Purkinje cell spiking elevates global Ca2+, facilitating CaV2.1 opening via CDF. Increased CaV2.1 activity in turn activates colocalized SK channels, resulting in stronger membrane hyperpolarization that then reduces spiking rate. B, similarly, decreased spiking rate leads to lower global Ca2+, reduced CDF, and less SK activation, finally resulting in weaker hyperpolarization and restoration of spiking rate. C, Ca2+ dose–response curves for CDF (continuous black line) and neurotransmitter secretion (red line). Additionally, Ca2+ sensitivity of paired-pulse facilitation due to residual Ca2+ (Regehr et al. 1994) (dashed line) shows an additional mechanism specifying the extent of transmitter release. D, generic nerve terminal is modelled as a sphere with CaV2.1 channels uniformly expressed on the membrane. A thin shell compartment adjacent to the membrane forms the nanodomain within which the CDF Ca2+ sensor CaM, which acts as a channel subunit, actually senses Ca2+. Ca2+ within the shell compartment can diffuse to and from the much larger cytosolic compartment. The cytosolic compartment is in turn connected to a third and much larger compartment representing the axon branch itself. The Ca2+ concentration within this axonal compartment is set at a fixed resting level, and thus serves to returns Ca2+ within the terminal back to its resting levels after periods of activity. The parameters for this system are: rsphere = 8 μm, rcyto = 7.9 μm, Nchan = 60,000, ksc = kcs = 6 × 10−13 dm3 ms−1, and kefflux = 6 × 10−14 dm3 ms−1, with the parameters adjusted such that simulations in E match data from Cuttle et al. (1998). Note that the simulated volume is larger than physical terminals because Ca2+ buffers are not explicitly modelled here. E, simulations of the system in D in response to 100 Hz step depolarizations to −20 mV. CaV2.1 currents are elicited (bottom row) by the voltage stimulation (top row), which results in a build-up of Ca2+ in both the shell (second row) and cytosolic (third row) compartments. The increased Ca2+ induces CDF of CaV2.1 channels, resulting in gradual amplification of ICa, Cashell and Cacyto. F, CDF at steady state is inversely related to stimulation frequency (blue circles and continuous line), largely due to frequency-dependent accumulation of residual Ca2+ (100 Hz example in red).