Figure 2. Generation of HERV-K env-specific CAR+ T cells.
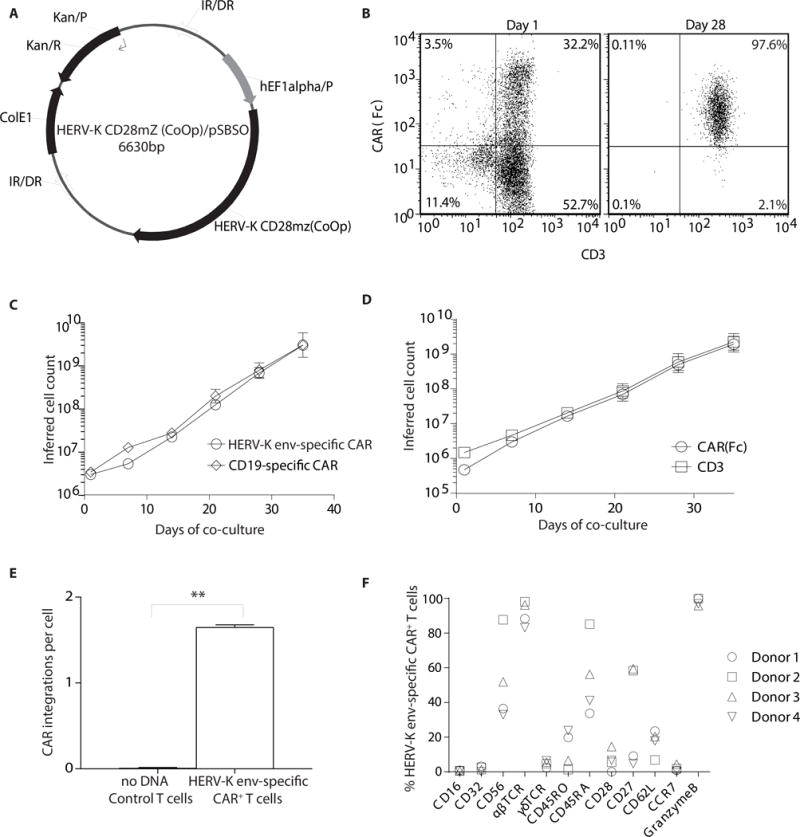
(A) SB-derived DNA transposon (HERV-K CD28mZ (CoOp)/pSBSO) designed to express HERV-K env-specific CAR. Abbreviations: IR/DR: inverted repeat/direct repeat, hEF-1alpha/p: human elongation factor-1α hybrid promoter, HERV-K CD28mZ (CoOp): codon-optimzed HERV-K envelope scFv, ColE1: E. coli origin of replication, Kan/R: kanamycin resistance, Kan/p: kanamycin resistance promoter. PBMCs (n = 4) were electroporated with SB plasmids, HERV-K-specific CAR and SB11 transposase and co-cultured with AaPCs with cytokines. (B) Representative flow plots of transient (day 1) and stable (day 28) CAR (binding of Fc-specific mAb) and CD3 expression on HERV-K env-specific CAR+ T-cell cultures. (C) Rate of expansion of total HERV-K env-specific CAR+ T cells and CD19-specific CAR+ T cells propagated on aAPC and cytokines. There was no difference in the kinetics of expansion. (D) Rate of expansion of CD3+ and CAR+ HERV-K env-specific T cells propagated on aAPC and cytokines. (E) Measurement of the copy number of CAR (transposon) in T-cell genome after electroporation and propagation. (F) Multiparameter flow cytometry to evaluate the phenotype of HERV-K env-specific CAR+ T cells. Each symbol represents individual donor. Data represented as mean or mean ± SD (n = 4); unpaired Student’s t-test; **p < 0.01.
