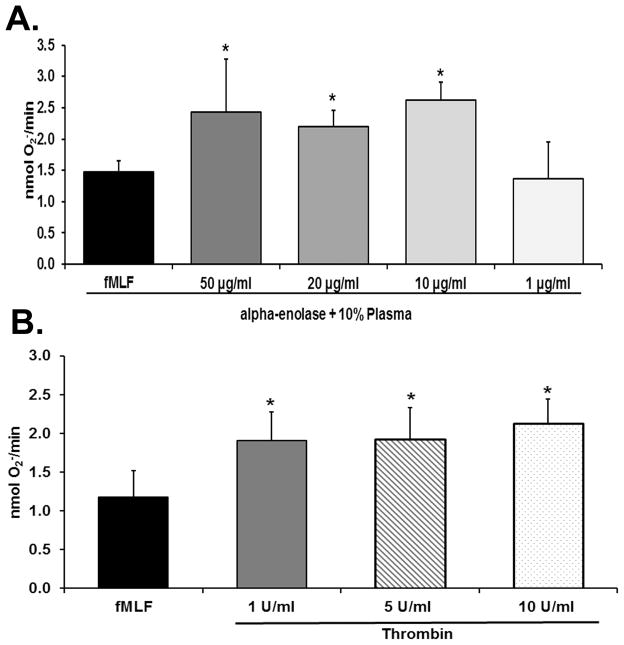
Figure 3. α-enolase and thrombin prime the PMN oxidase.
In both panels the maximal rate of superoxide anion production (O2− nmol/min) is depicted as a function of treatment group. Panel A demonstrates that α-enolase at concentration of 10–50 μg/ml caused significant priming of the formyl-Met-Leu-Phe (fMLF)-activated respiratory burst (*=p<0.05 vs. buffer-primed/fMLF-activated PMNs, n=6). Concentrations of α-enolase of 1 μg/ml did not cause PMN priming. Panel B shows that thrombin at concentrations of 1–10 U/ml significantly primed the fMLF-activated respiratory burst whereas lesser concentration of thrombin did not (*=p<0.05 vs. buffer primed/fMLF-activated PMNs, n=6).