Abstract
Background
Steaming of ginseng is known to change its chemical composition and biological activity. This study was carried out to investigate the effect of different steaming time-scales on chemical constituents and antiproliferative activity of Vietnamese ginseng (VG).
Methods
VG was steamed at 105°C for 2–20 h. Its saponin constituents and antiproliferative activity were studied. The similarity of chemical compositions between steamed samples at 105°C and 120°C were compared.
Results
Most protopanaxadiol and protopanaxatriol ginsenosides lost the sugar moiety at the C-20 position with 10–14 h steaming at 105°C and changed to their less polar analogues. However, ocotillol (OCT) ginsenosides were reasonably stable to steaming process. Antiproliferative activity against A549 lung cancer cells was increased on steaming and reached its plateau after 12 h steaming.
Conclusion
Steaming VG at 105°C showed a similar tendency of chemical degradation to the steaming VG at 120°C except the slower rate of reaction. Its rate was about one-third of the steaming at 120°C.
Keywords: antiproliferation, ginsenoside Panax vietnamensis, steaming, Vietnamese ginseng
1. Introduction
Panax vietnamensis Ha et Grushv. or Vietnamese ginseng (VG) was reported in 1973, and is the most recently reported Panax plant [1,2]. VG contains ginsenosides as other Panax plants, but contains not only protopanaxadiol (PPD) and protopanaxatriol (PPT) ginsenosides, but also contains ocotillol (OCT) saponins, such as majonoside R1 (M-R1), majonoside R2 (M-R2), vinaginsenoside R1 (V-R1), and vinaginsenoside R2 (V-R2) in high yields [3,4]. In particular, M-R2 is a major saponin in VG and plays an important role in pharmacological effects on the central nervous system [5–7].
Panax ginseng or Korean ginseng (KG) has been regarded as an important and valuable herbal medicine for thousands years. Red ginseng is a traditional steamed preparation of P. ginseng. Red ginseng shows enhanced pharmacological activities over white ginseng in most cases. The difference in biological activities of white and red ginsengs results from the change of their chemical constituents that occur during the steaming process [8]. It has been reported that the steaming of KG at higher temperature induces the change in its chemical composition and increase of its biological activity [9–11].
Recently, we reported that steaming VG at 120°C induces the modification of saponin constituents and enhancement of its biological activity [12]. However, the temperature of 120°C is slightly high for the processing. Therefore, as a part of our continuing study on processed VG, the processing temperature at 105°C for VG was studied on the saponins composition and antiproliferative activity.
2. Materials and methods
2.1. Materials and reagents
VG was collected in 2010 from Quangnam Province, Vietnam. The voucher specimen was deposited at the herbarium of College of Pharmacy, Seoul National University (SNUP-2012-A-01).
A Perkin Elmer series 200 HPLC (high performance liquid chromatography; Perkin Elmer, Inc., Waltham, MA, USA) system equipped with Alltech ELSD 2000 (Evaporative Light Scattering Detector; Alltech, Deerfield, IL, USA) and Sunfire C18 column (250 mm × 4.6 mm. i.d., 5 μm); (Waters Corporation, Milford, MA, USA) was used for HPLC analysis. MicroToF-QII LC/MS (Bruker Daltonics, Bremen, Germany) was used for the LC/MS analysis. SpectraMax 340PC384 microplate reader (Molecular Devices, Sunnyvale, CA, USA) was used to measure the absorbance of the samples.
A549 lung cancer cell line was purchased from the American Type Culture Collection (ATCC, Manassas, VA, USA). DMEM/F12 media, fetal bovine serum (FBS), penicillin/streptomycin antibiotics, and phosphate buffer saline (PBS) were purchased from Gibco (Grand Island, NY, USA). 3-(4,5-Dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide (MTT) was purchased from Amresco (Solon, OH, USA) and DMSO was purchased from Sigma–Aldrich (St. Louis, MO, USA). Solvents for HPLC were purchased from Duksan (Ansan, Korea). Ginsenoside standards were isolated and identified from KG and VG in our laboratories [3,9].
2.2. Sample preparation
Dried underground part of VG, including radix, rhizome, and hairy root, was pulverized and sieved to get the powder of 355–425 μm. Each sample (150 mg) was placed into a stainless steel vessel with 1.5 mL of distilled water. The vessel was closed tightly and heated in an oven at 105°C for 2 h, 4 h, 6 h, 8 h, 10 h, 12 h, 14 h, 16 h, 18 h, or 20 h. After heating, the steamed samples were lyophilized and were extracted three times by ultrasonication at 65°C for 1.5 h, 1 h, and 0.5 h, using 10 mL, 10 mL, and 5 mL of MeOH, respectively. The combined extract was made up to 25 mL with MeOH.
2.2.1. For HPLC analysis
Two mL of the extract of each sample was dried under nitrogen stream. The residue was dissolved in 1 mL of MeOH and then filtered through a 0.45-μm membrane filter prior to HPLC analysis.
2.2.2. For cell proliferation analysis
The MeOH extract of each sample was dried under nitrogen stream, and dissolved in DMEM/F12 media containing 0.1% DMSO to get different concentrations for the cell proliferation analysis.
2.3. HPLC analysis
A previously reported method [12,13] was applied for the HPLC analysis of ginsenosides with slight modification: water (A) and acetonitrile (B): 0–1 min (18% B), 1–5 min (18→25% B), 5–25 min (25→32% B), 25–29 min (32% B), 29–40 min (32→38% B), 40–45 min (38→49.5% B), 45–59 min (49.5→100% B), and 59–69 min (100% B). The flow rate was 1 mL/min and the injection volume was 10 μL. ELSD was set to a probe temperature of 80°C and the nebulizer gas (N2) was adjusted to 1.5 L/min.
2.4. Cell proliferation analysis
A549 lung cancer cells were grown in DMEM/F12 medium supplemented with 10% fetal bovine serum and 1% antibiotics in a humidified atmosphere of 5% CO2 at 37°C.
Antiproliferative activity was measured by a previously reported method [14]. A549 lung cancer cells (200 μL) at 5 × 104 cells/mL were seeded in 96-well plates and incubated for 24 h. Then 200 μL of VG extract in DMEM/F12 media containing 0.1% DMSO was added to each well to make a final concentration 0.75-, 1.5-, and 3-mg of dried VG/mL of final medium. After incubation for 24 h, the supernatant was removed, 50 μL MTT in PBS (4 mg/mL) was added into each well, and incubated for 60 min. The supernatant was removed and 100 μL DMSO was added into each well, and then incubated for 30 min to dissolve the purple formazan crystals formed. The absorbance was measured at 570 nm by microplate reader.
2.5. Statistical analysis
The data are presented as the mean ± standard deviation. Data were analyzed by Student t test to compare two groups using SPSS version 21.0 (IBM Corp., Armonk, NY, USA). A p value < 0.05 was considered statistically significant.
3. Results and discussion
3.1. Effect of steaming duration on chemical constituents
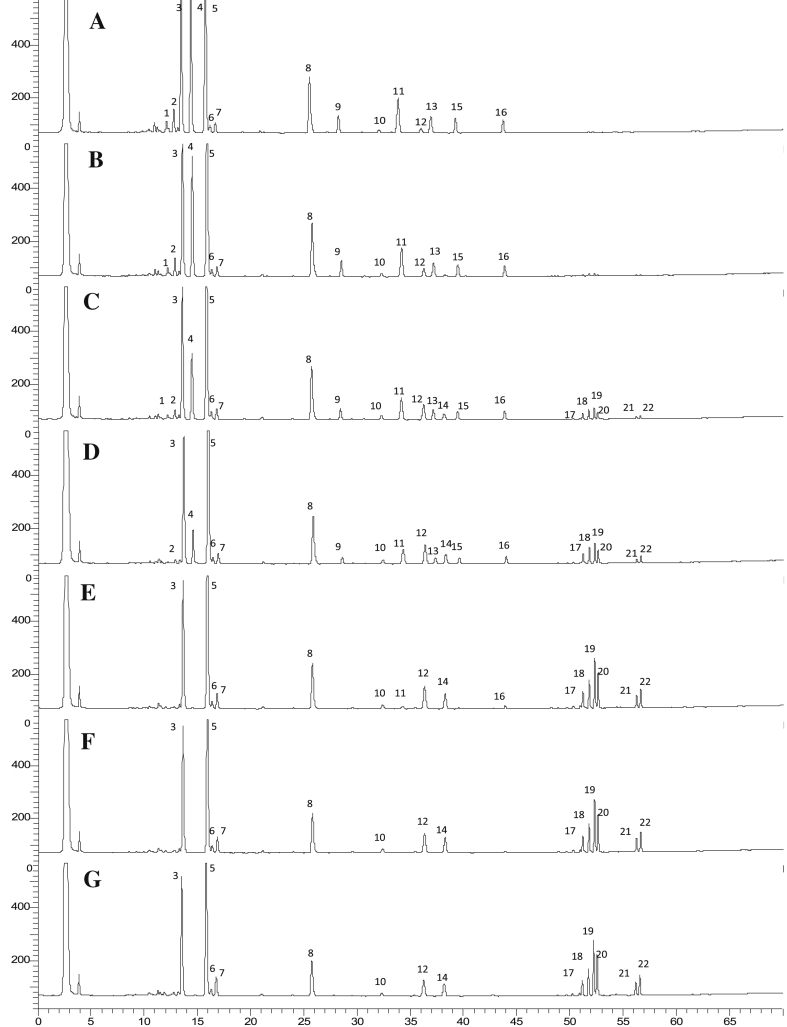
Fig 1 shows the typical HPLC chromatograms of VG steamed at 105°C. The peak intensities of polar saponins, which appeared prior to 45 min, were decreased by the steaming process while those of less polar ginsenosides, which appeared after 45 min, were increased as shown in Fig. 1. This result is in accordance with our previous report in processed VG which was steamed at 120°C [12]. The quantitative results are summarized in Table 1. The contents of unknown peaks 1–2 and 3–5 were calculated by comparing ELSD responses to M-R2 and G-Rb1, respectively.
Fig. 1.
Typical HPLC-evaporative light scattering detector chromatograms. Raw (A) and steamed Vietnamese ginseng at 105°C for 2 h (B), 4 h (C), 8 h (D), 12 h (E), 16 h (F), and 20 h (G). Peaks: 1, unknown 1; 2, unknown 2; 3, M-R1; 4, G-Rg1 + G-Re; 5, M-R2; 6, V-R11; 7, P-RT4; 8, V-R2 + V-R1; 9, unknown 3; 10, unknown 4; 11, G-Rb1; 12, 20(S) G-Rh1; 13, unknown 5; 14, 20(R) G-Rh1; 15, G-Rb2; 16, G-Rd; 17, G-Rk3; 18, G-Rh4; 19, 20(S) G-Rg3; 20, 20(R) G-Rg3; 21, G-Rk1; 22, G-Rg5. HPLC, high performance liquid chromatography.
Table 1.
Contents1) of saponins in raw and processed vietnamese ginseng
| Peak No.2) | Saponin | Raw (0 h) | Steaming time |
|||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 2 h | 4 h | 6 h | 8 h | 10 h | 12 h | 14 h | 16 h | 18 h | 20 h | |||
| 1 | Unknown 13) | 5.0 | 3.8 | 2.5 | 1.5 | 0.9 | 0.6 | 0.8 | 1.2 | 1.2 | 1.4 | 1.6 |
| 2 | Unknown 23) | 8.9 | 6.9 | 4.6 | 3.0 | 2.2 | 1.3 | 1.2 | 1.8 | 1.5 | 1.5 | 1.7 |
| 3 | M-R1 | 40.4 | 39.0 | 36.5 | 36.2 | 36.2 | 35.0 | 35.0 | 34.1 | 34.6 | 33.6 | 33.2 |
| 4 | G-Re + G-Rg14) | 42.3 | 34.8 | 21.0 | 14.3 | 11.8 | 2.0 | N.D | N.D | N.D | N.D | N.D |
| 5 | M-R2 | 53.5 | 52.5 | 51.5 | 51.1 | 49.0 | 49.7 | 49.1 | 45.5 | 46.6 | 48.6 | 45.2 |
| 6 | V-R11 | 3.9 | 3.8 | 3.8 | 4.2 | 3.7 | 4.4 | 4.0 | 3.9 | 4.0 | 4.2 | 3.9 |
| 7 | P-RT4 | 5.2 | 4.8 | 5.1 | 5.6 | 5.6 | 6.9 | 6.9 | 7.4 | 7.3 | 8.4 | 8.9 |
| 8 | V-R1 + V-R25) | 29.7 | 28.3 | 27.6 | 26.8 | 24.6 | 23.8 | 23.0 | 20.8 | 20.9 | 21.9 | 20.4 |
| 9 | Unknown 33) | 10.8 | 9.6 | 7.4 | 5.6 | 4.8 | 1.4 | N.D | N.D | N.D | N.D | N.D |
| 10 | Unknown 43) | 3.6 | 3.8 | 4.0 | 4.8 | 4.1 | 4.8 | 4.0 | 3.4 | 3.9 | 3.7 | 3.6 |
| 11 | G-Rb1 | 19.9 | 18.1 | 14.3 | 12.6 | 10.9 | 5.0 | 2.6 | N.D | N.D | N.D | N.D |
| 12 | 20(S) G-Rh1 | 5.1 | 7.0 | 11.0 | 12.8 | 13.1 | 14.4 | 14.8 | 13.3 | 13.4 | 13.0 | 11.3 |
| 13 | Unknown 53) | 11.2 | 9.4 | 7.2 | 5.8 | 5.6 | 1.8 | 1.1 | N.D | N.D | N.D | N.D |
| 14 | 20(R) G-Rh1 | N.D6) | 2.5 | 5.4 | 7.5 | 8.1 | 9.8 | 10.5 | 10.1 | 10.5 | 10.7 | 9.4 |
| 15 | G-Rb2 | 9.3 | 7.9 | 6.4 | 6.0 | 5.1 | 2.0 | 2.1 | N.D | N.D | N.D | N.D |
| 16 | G-Rd | 8.2 | 7.1 | 6.0 | 5.9 | 5.8 | 3.5 | 2.4 | 1.5 | 1.8 | 1.9 | 1.1 |
| 17 | G-Rk3 | N.D | 0.7 | 2.3 | 3.3 | 3.5 | 5.2 | 5.5 | 5.7 | 5.6 | 5.9 | 5.4 |
| 18 | G-Rh4 | N.D | 1.1 | 3.5 | 4.8 | 5.1 | 7.2 | 8.1 | 7.9 | 8.3 | 8.4 | 8.0 |
| 19 | 20(S) G-Rg3 | N.D | 1.1 | 3.6 | 5.4 | 6.0 | 10.4 | 12.7 | 12.8 | 13.2 | 14.2 | 13.9 |
| 20 | 20(R) G-Rg3 | N.D | 0.6 | 2.5 | 3.9 | 4.5 | 7.9 | 9.5 | 10.1 | 10.1 | 11.1 | 10.9 |
| 21 | G-Rk1 | N.D | 0.3 | 1.1 | 1.6 | 1.9 | 3.6 | 4.5 | 4.6 | 4.7 | 5.1 | 5.0 |
| 22 | G-Rg5 | N.D | 0.5 | 1.5 | 2.2 | 2.6 | 4.8 | 6.0 | 6.1 | 6.6 | 6.5 | 6.8 |
| PPD(1)7) | 37.3 | 33.0 | 26.7 | 24.5 | 21.7 | 10.5 | 7.1 | 1.5 | 1.8 | 1.9 | 1.1 | |
| PPT (1)8) | 42.3 | 34.8 | 21.0 | 14.3 | 11.8 | 2.0 | N.D | N.D | N.D | N.D | N.D | |
| PPD(2)9) | N.D | 2.6 | 8.7 | 13.2 | 15.0 | 26.7 | 32.6 | 33.7 | 34.6 | 36.9 | 36.6 | |
| PPT(2)10) | 5.1 | 11.3 | 22.2 | 28.4 | 29.8 | 36.7 | 39.0 | 37.0 | 37.8 | 38.0 | 34.1 | |
| OCT11) | 132.8 | 128.4 | 124.6 | 123.9 | 119.0 | 119.8 | 118.1 | 111.7 | 113.4 | 116.7 | 111.6 | |
| Sum | 256.8 | 243.5 | 229.0 | 225.1 | 215.0 | 205.7 | 203.8 | 190.3 | 194.3 | 200.0 | 190.3 | |
OCT, ocotillol; PPD, protopanaxadiol; PPT, protopanaxatriol; VG, Vietnamese ginseng; ELSD, Evaporative Light Scattering Detector
Result are expressed as mg/(g dried VG).
Peak No. in Fig. 3.
Concentrations of unknown 1–2 and 3–5 were calculated by comparing ELSD responses to standard N-R1 and G-Rb1, respectively.
Calculated as G-Rg1.
Calculated as V-R2
N.D. not detected
Polar PPD-type ginsenosides: G-Rb1, G-Rb2, and G-Rd
Polar PPT-type ginsenosides: G-Re and G-Rg1.
Less polar PPD-type ginsenosides: 20(S) G-Rg3, 20(R) G-Rg3, G-Rk1, and G-Rg5
Less polar PPT-type ginsenosides: 20(S) G-Rh1, 20(R) G-Rh1, G-Rk3, and G-Rh4.
Ocotillol saponins: M-R1, M-R2, V-R1, and V-R2.
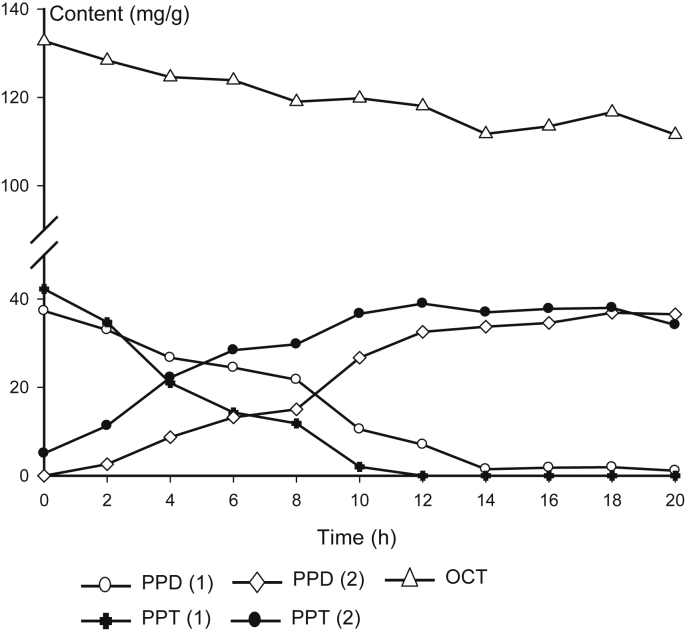
Degradation and formation of PPD, PPT, and OCT ginsenosides are summarized in Fig. 2. Polar PPT ginsenosides (G-Rg1 and G-Re) were degraded faster than polar PPD ginsenosides. They were not detected at 12 h when less polar PPT ginsenosides reached their plateau. Among polar PPD ginsenosides, G-Rd was relatively more stable than G-Rb1 and G-Rb2. G-Rd remained until 20 h and fewer polar PPD ginsenosides were gradually increased until 20 h.
Fig. 2.
Change of content of protopanaxadiol (PPD), protopanaxatriol (PPT), and ocotillol (OCT) saponins during the steaming process. PPD (1): G-Rb1, G-Rb2, and G-Rd; PPT (1): G-Re and G-Rg1; PPD (2): 20(S) G-Rg3, 20(R) G-Rg3, G-Rk1, and G-Rg5; PPT (2): 20(S) G-Rh1, 20(R) G-Rh1, G-Rk3, and G-Rh4. OCT: M-R1, M-R2, V-R1, and V-R2.
The contents of OCT saponins were not changed much as they do not have heat-labile C-20 glycoside. About 84% of OCT saponins were remained even after 20 h of steaming. The content of P-RT4 was increased by 70% after 20 h. It may infer that P-RT4 was produced by the deglycosylation of M-R1 and M-R2 at C-6 position by the steaming.
The total saponin content in dried VG was 25.68%, which was decreased to 19.03% after 20 h steaming (Table 1). This can be explained by the decrease of molecular mass due to the loss of sugar moiety and the degradation to unknown compounds.
3.2. Antiproliferative effect on lung cancer cells.
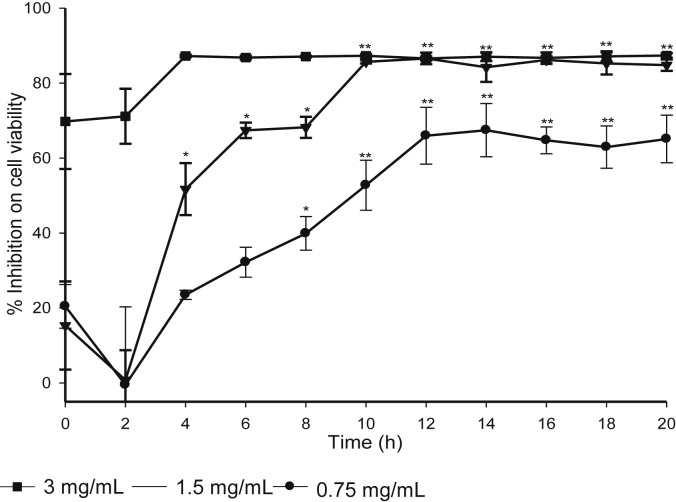
The change in antiproliferative activity of processed VG on lung cancer cell line is shown in Fig. 3. The antiproliferative activity was increased significantly upon steaming and reached its maximum at 12 h. It is noteworthy that the content of less polar ginsenosides was reached its plateau at 12 h (Fig. 3), which suggests a relationship between the antiproliferative activity and the content of less polar ginsenosides, in particular G-Rg3, G-Rg5, and G-Rk1 [9,15].
Fig. 3.
Antiproliferative activity of Vietnamese ginseng on lung cancer cell at different times of steaming. Results are expressed as mean ± standard deviation (n = 3), *p < 0.05, **p < 0.01 compared with raw sample (Student t test). The concentrations are expressed as a dry weight of Vietnamese ginseng in mL of final medium.
3.3. Comparison of steaming temperature of 105°C and 120°C.
Steaming VG at 105°C showed similar tendency to the steaming VG at 120°C in the degradation of ginsenosides and the formation of less polar ginsenosides except its rate was slower. Table 2 shows similarity index (SI) between the chromatograms of two different processing temperatures. SI was calculated using the concentration ratio as follows:
| (1) |
where, Csi and Cti are the concentrations of ith component in samples steamed at 105°C and 120°C, respectively. Data in the previous report were used for the concentration of ginsenosides steamed at 120°C [12]. The ginsenoside pattern of steamed VG at 120°C for 2 h (120-2 in Table 2) was similar to those of steamed VG at 105°C for 4–8 h (105-4, 105-6, and 105-8 in Table 2), and the patterns of steamed VG at 120°C for 4 h and 6 h were close to those of steamed VG at 105°C for 10–12 h and 14–20 h, respectively, as shown in Table 2. It is known that the reaction rate increases 2–3 fold when the temperature increase by 10°C. This phenomenon was also observed in this study. The rate of degradation of ginsenosides was increased about three fold when the temperature increased by 15°C.
Table 2.
Similarity index (SI) between the chromatograms of two different processing temperatures
| Degree–hour | 120–0 | 120–2 | 120–4 | 120–6 | 120–8 | 120–10 |
|---|---|---|---|---|---|---|
| 105–0 | 0.903 | 0.693 | 0.530 | 0.468 | 0.420 | 0.421 |
| 105–2 | 0.902 | 0.753 | 0.585 | 0.525 | 0.480 | 0.478 |
| 105–4 | 0.837 | 0.861 | 0.687 | 0.632 | 0.592 | 0.585 |
| 105–6 | 0.789 | 0.903 | 0.743 | 0.691 | 0.652 | 0.635 |
| 105–8 | 0.774 | 0.918 | 0.774 | 0.722 | 0.683 | 0.664 |
| 105–10 | 0.663 | 0.819 | 0.883 | 0.835 | 0.796 | 0.772 |
| 105–12 | 0.628 | 0.785 | 0.903 | 0.877 | 0.836 | 0.815 |
| 105–14 | 0.602 | 0.780 | 0.900 | 0.905 | 0.872 | 0.851 |
| 105–16 | 0.601 | 0.774 | 0.902 | 0.906 | 0.868 | 0.852 |
| 105–18 | 0.594 | 0.762 | 0.901 | 0.906 | 0.863 | 0.857 |
| 105–20 | 0.596 | 0.770 | 0.901 | 0.909 | 0.881 | 0.869 |
G-Rh1 and G-Rg3 have two isomers of 20(S)- and 20(R)-epimers. 20(S)-form of G-Rh1 was predominant at the beginning since 20(S) is a natural form. However 20(R)-form was gradually increased on heating. 20(R)/20(S) ratio for G-Rh1 was 0.36 at 2 h, which was increased up to 0.83 at 20 h. G-Rg3 showed similar phenomena. 20(R)/20(S) ratio for G-Rg3 was 0.55 at 2 h, which was increased up to 0.78 at 20 h. The same result was observed in our previous report [12]. 20(R)/20(S) ratio for G-Rh1 after 2 h steaming at 120°C was 0.52, which was increased to 1.98 at 20 h. 20(R)/20(S) ratio for G-Rg3 after 2 h at 120°C were 0.78, which was increased to 1.11 at 20 h. The ratios of G-Rk1/G-Rg5 and G-Rk3/G-Rh4, and other stereoisomer pairs, were not changed much upon steaming.
4. Conclusion
Steaming VG at 105°C showed similar tendency of change in saponin constituents and antiproliferative activity to the steaming at 120°C. Similarity study showed that the rate of degradation of ginsenoside was increased about three fold when the temperature increased from 105°C to 120°C. Antiproliferative activity against A549 lung cancer cell line reached maximum at 12 h steaming. The ratios of stereoisomers of 20(R)/20(S) G-Rg3 and G-Rh1 were increased upon steaming time, while the ratios of Rk1/Rg5 and Rk3/Rh4 were not significantly changed.
Conflicts of interest
All contributing authors declare no conflicts of interest.
Acknowledgments
This work was supported by the grant from Ministry of Science and Technology of The Socialist Republic of Vietnam (No. KC 10.25/11-15), Ministry of Education, Science and Technology (No. 2012M3A9C4048796), and Rural Development Administration of Korea (No. PJ008202).
Footnotes
This is an Open Access article distributed under the terms of the Creative Commons Attribution Non-Commercial License (http://creativecommons.org/licenses/by-nc/3.0) which permits unrestricted non-commercial use, distribution, and reproduction in any medium, provided the original work is properly cited
Contributor Information
Jeong Hill Park, Email: hillpark@snu.ac.kr.
Minh Duc Nguyen, Email: ducng@hcm.vnn.vn.
References
- 1.Nguyen T.N. Study on Panax vietnamensis Ha et Grushv., Araliaceace. Botany, Tissue culture-Chemistry-Biological properties. Herba Pol. 1989;35:1–229. [Google Scholar]
- 2.Lutomski J., Nguyen T.N. Study on species of Panax growing in Vietnam. I. Comparative study on the saponins from Panax K5VN and Panax ginseng by TLC. Herba Pol. 1976;1:23–27. [Google Scholar]
- 3.Nguyen M.D., Kasai R., Ohtani K., Ito A., Nguyen T.N., Yamasaki K., Tanaka O. Saponins from Vietnamese Ginseng, Panax vietnamensis Ha et Grushv. Collected in central Vietnam. II. Chem Pharm Bull. 1994;42:115–122. doi: 10.1248/cpb.42.115. [DOI] [PubMed] [Google Scholar]
- 4.Nguyen M.D., Kasai R., Yamasaki K., Nguyen T.N., Tanaka O. New dammarane saponins from Vietnamese ginseng. In: Chong-Ren Y., Osamu T., editors. vol. 6. Elsevier; Philadelphia, PA: 1999. pp. 77–82. (Studies in plant science). [Google Scholar]
- 5.Nguyen T.T.H., Matsumoto K., Watanabe H. The antistress effect of majonoside-R2, a major saponin component of Vietnamese ginseng: neuronal mechanisms of action. Methods Find Exp Clin Pharmacol. 1998;20:65–76. doi: 10.1358/mf.1998.20.1.485634. [DOI] [PubMed] [Google Scholar]
- 6.Nguyen T.T.H., Matsumoto K., Yamasaki K., Nguyen M.D., Nguyen T.N. Majonoside-R2, a major constituent of Vietnamese ginseng, attenuates opioid-induced antinociception. Pharmacol Biochem Behav. 1997;57:285–291. doi: 10.1016/s0091-3057(96)00348-6. [DOI] [PubMed] [Google Scholar]
- 7.Shi W., Wang Y., Li J., Zhang H., Ding L. Investigation of ginsenosides in different parts and ages of Panax ginseng. Food Chem. 2007;102:664–668. [Google Scholar]
- 8.Kim U., Park M.H., Kim D.H., Yoo H.H. Metabolite profiling of ginsenoside Re in rat urine and faeces after oral administration. Food Chem. 2013;136:1364–1369. doi: 10.1016/j.foodchem.2012.09.050. [DOI] [PubMed] [Google Scholar]
- 9.Kim W.Y., Kim J.M., Han S.B., Lee S.K., Kim N.D., Park M.K., Kim C.K., Park J.H. Steaming of ginseng at high temperature enhances biological activity. J Nat Prod. 2000;63:1702–1704. doi: 10.1021/np990152b. [DOI] [PubMed] [Google Scholar]
- 10.Kim E.J., Oh H.A., Choi H.J., Park J.H., Kim D.H., Kim N.J. Heat-processed ginseng saponin ameliorates the adenine-induced renal failure in rats. J Ginseng Res. 2013;37:87–93. doi: 10.5142/jgr.2013.37.87. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Kang K.S., Ham J., Kim Y.J., Park J.H., Cho E.J., Yamabe N. Heat-processed Panax ginseng and diabetic renal damage: active components and action mechanism. J Ginseng Res. 2013;37:379–388. doi: 10.5142/jgr.2013.37.379. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Le T.H.V., Lee S.Y., Kim T.R., Kim J.Y., Kwon S.W., Nguyen N.K., Park J.H., Nguyen M.D. Processed Vietnamese ginseng: preliminary results in chemistry and biological activity. J Ginseng Res. 2014;38:154–159. doi: 10.1016/j.jgr.2013.11.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Kwon S.W., Han S.B., Park I.H., Kim J.M., Park M.K., Park J.H. Liquid chromatographic determination of less polar ginsenosides in processed ginseng. J Chromatogr A. 2001;921:335–339. doi: 10.1016/s0021-9673(01)00869-x. [DOI] [PubMed] [Google Scholar]
- 14.Chen G., Yang M., Nong S., Yang X., Ling Y., Wang D., Wang X., Zhang W. Microbial transformation of 20(S)-protopanaxadiol by Absidia corymbifera. Cytotoxic activity of the metabolites against human prostate cancer cells. Fitoterapia. 2013;84:6–10. doi: 10.1016/j.fitote.2012.09.018. [DOI] [PubMed] [Google Scholar]
- 15.Park I.H., Kwon S.W., Lee Y.J., Cho S.Y., Park M.K., Park J.H. Cytotoxic dammarane glycosides from processed ginseng. Chem Pharm Bull. 2002;50:538–540. doi: 10.1248/cpb.50.538. [DOI] [PubMed] [Google Scholar]