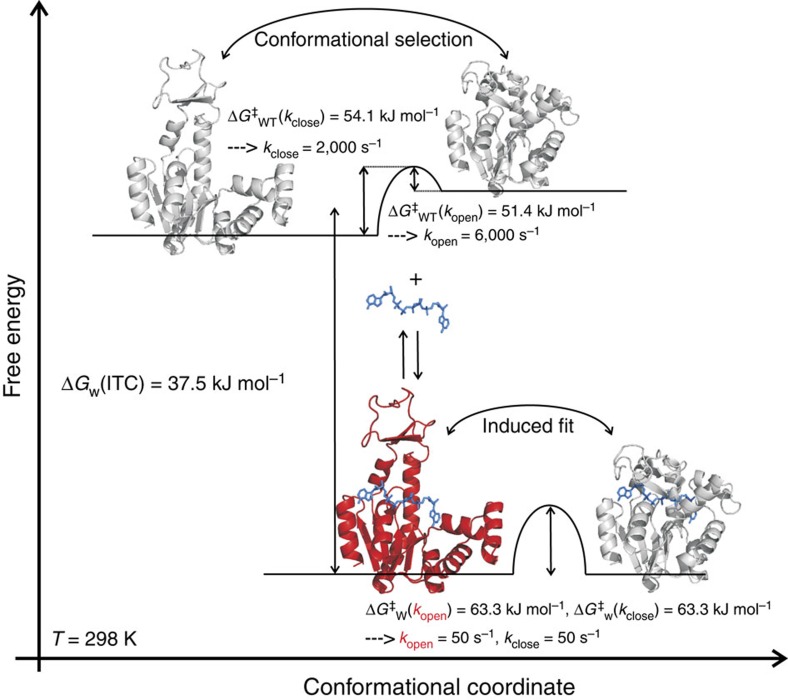
Figure 5. The high-energy state is crucial for the catalytic mechanism of AdK.
The state shown in red corresponds to the directly observed high-energy state of Y171W and is modelled from known structures (1AKE and 4AKE). This state completes the description of conformations required for AdK catalysis. Relative ground state free energies and activation barriers, ΔG‡, are indicated for apo-11 and substrate-bound AdK. The binding of Ap5a to Y171W lowers the free energy of the system by 37.5 kJ mol−1. Kinetic rate constants are displayed for the dynamic interconversion between structural states. Taken together, the data show that population of closed AdK conformations is accomplished through conformational selection29 in the apo state and induced fit in the presence of substrate27. Subscripts ‘WT' and ‘W' correspond to wild type and Y171W, respectively. Although no direct high-resolution structural data exist for the closed apo structure, indirect evidence from ensemble49 and single-molecule11 fluorescence resonance energy transfer experiments and chemical shifts13 points towards that compact ‘closed-like' structures are sampled by AdK in the absence of substrate.