Figure 2.
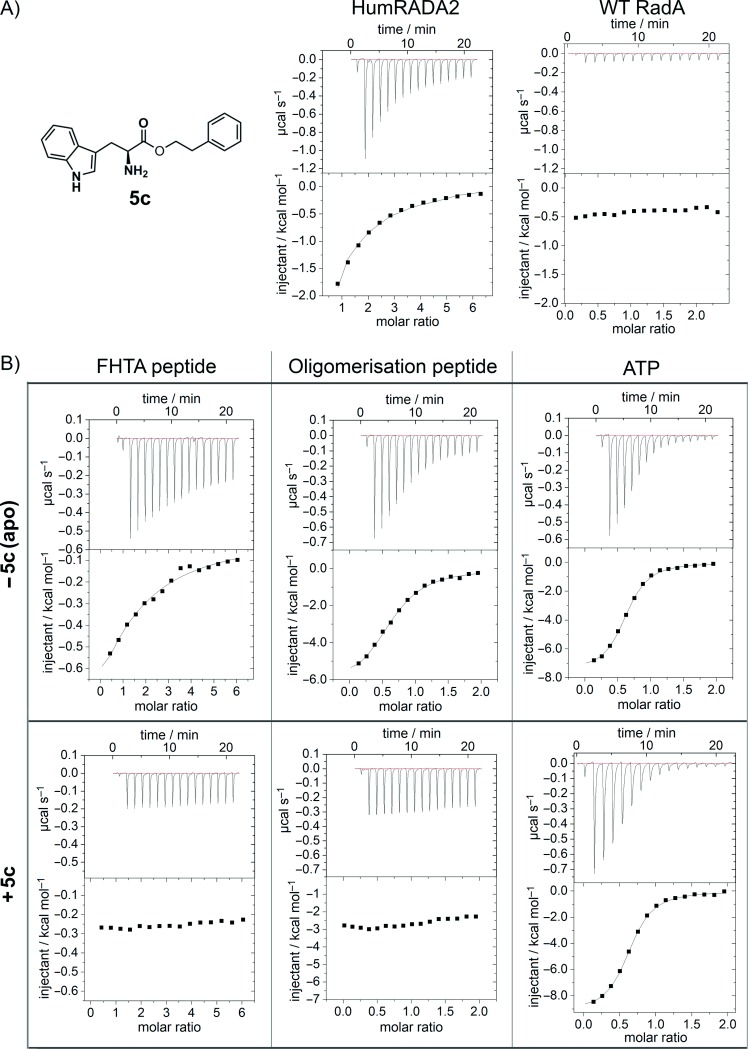
A) Structure of compound 5 c, and binding directly to HumRADA2 (left, KD=35 μm) and showing no binding to the wild-type RadA (right). B) Competitive ITC experiments to HumRADA2 indicate the binding site of 5 c. Top row: ITC experiments in the absence of 5 c determined the KD values of Ac-FHTA-NH2, the RadA oligomerisation peptide, and ATP to be 290, 7.0, and 2.5 μm, respectively. Bottom row: ITC of Ac-FHTA-NH2, RadA oligomerisation peptide, and ATP in the presence of 200 μm 5 c shows competition with the FxxA binding site, but not the ATP binding site.
