Table 4.
Experimental and DFT computed EPR parameters for radical cations of aryloxy-, arylthio-acetic acids and esters.[a]
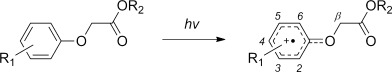 | |||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Entry | Radical cation | R1 | R2 | Method (expt./DFT[b]) | g-factor | a(2 Hβ)[c] | a(H2) | a(H3) | a(H4) | a(H5) | a(H6) |
| 1 | 4b.+ | 4-Me | H | Expt. | 2.0036 | 6.3 | 3.5 | 1.0 | 14.3 (3 H) | 1.0 | 2.6 |
| 2 | 4b.+ | 4-Me | H | DFT | – | 7.8 | −4.2 | 0.6 | 16.7 (3 H) | −1.2 | −2.5 |
| 3 | 4c.+ | 4-tBu | H | Expt. | 2.0030 | 6.7 | 3.4 | 0.7 | 0.8 (9 H) | 1.2 | 2.1 |
| 4 | 4c.+ | 4-tBu | H | DFT | – | 7.4 | −4.2 | 0.4 | 0.9 (9 H) | −1.6 | −2.0 |
| 5 | 4d.+ | 4-MeO | H | Expt. | 2.0039 | 2.6 | 1.9 | 0.8 | 1.7 (3 H)[c] | 4.8 | 0.8 |
| 6 | 4dt.+ | 4-MeO[d] | H | DFT | – | 6.4 | −2.7 | −1.2 | 4.4 (3 H)[c] | −3.1 | −0.9 |
| 7 | 4dc.+ | 4-MeO[d] | H | DFT | – | 6.1 | −1.3 | −1.3 | 4.2 (3 H)[c] | −2.6 | −2.6 |
| 8 | 15.+ | 4-MeO | Bn | Expt. | 2.0041 | 3.3 | 1.7 | 0.7 | 1.7 (3 H)[c] | 4.9 | 0.7 |
| 9 | 4h.+ | 3,4,5-triMeO | H | Expt. | 2.0040 | 5.2 | 1.3 | 0.4 (3 H)[c] | 0.9 (3 H)[c] | 0.4 (3 H)[c] | 0.9 |
| 10 | 4h.+ | 3,4,5-triMeO | H | DFT | – | 3.5 | −1.5 | 1.6 (3 H)[c] | 5.7 (3 H)[c] | 2.1 (3 H)[c] | −0.1 |
| 11 | 15.+ | 3,4,5-triMeO | Me | Expt. | 2.0037 | 4.1 | 3.2 | 0.4 (3 H)[c] | 1.1 (3 H)[c] | 0.7 (3 H)[c] | 1.1 |
| 12 | 4k.+ | 4-MeS | H | Expt. | – | 2.8 | 1.0 | 1.4 | 5.2 (3 H)[c] | 1.8 | 1.0 |
| 13 | 4kt.+ | 4-MeS | H | DFT | – | 4.8 | −1.7 | −1.1 | 7.1 (3 H)[c] | −2.5 | −0.5 |
| 14 | 4kc.+ | 4-MeS | H | DFT | – | 4.5 | −0.7 | −1.5 | 6.9 (3 H)[c] | −2.2 | −1.5 |
| 15 | 18.+[e] | – | Bn | Expt. | 2.0039 | 3.4 | 1.5 | 1.1 | 2.6 (2 H)[c] | 1.1 | 1.5 |
[a] Spectra in PhH solution at 300 K; hfs in Gauss. [b] DFT computations at the UB3LYP/6-311+G(2d,p) level of theory; note that the signs of hfs cannot be obtained from CW EPR spectra. [c] Hfs from beta-H-atoms are very sensitive to the dihedral angle subtended about the C–X bond by the three bonds linking the H-atom to the ring. The computed hfs are the values obtained for the single dihedral angle of the optimum geometry found by the DFT computation whilst the experimental EPR hfs are actually average values from all the dihedral angles the radicals access as they undergo internal motions in solution. This is the cause of the poor agreement. [d] Transoid and cisoid structures. [e] Dibenzyl ester of di-acid 8.
