Figure 3.
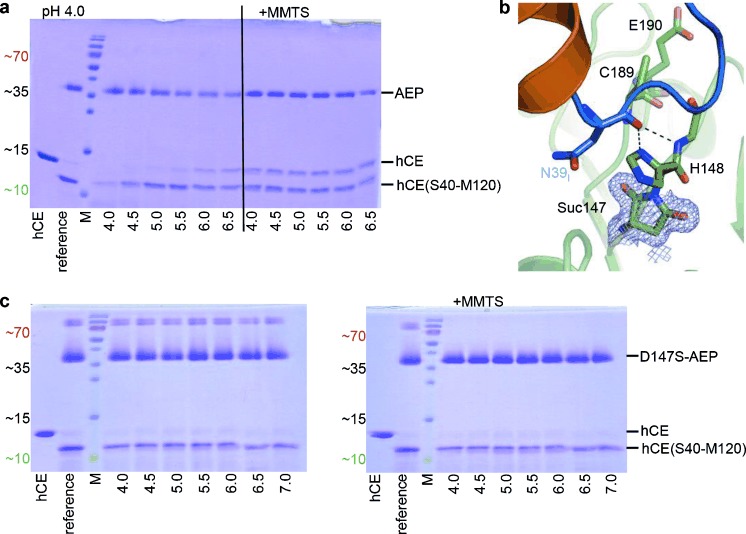
The ligase activity of legumain. a) Legumain (AEP) shows ligase activity at near-neutral pH that is not mediated by the catalytic Cys189. Upon incubation with AEP at pH 4.0, cystatin E (hCE) was completely converted to the Asn39I-processed form. Subsequent incubation at increasing pH values led to the reappearance of intact hCE, indicative of religation of the Asn39I–Ser40I peptide bond. Covalent modification of the catalytic Cys189 via addition of MMTS led to pH-independent resynthesis of hCE. b) Asp147 is present as succinimide in the AEP–hCE complex. An enlarged view of the AEP (green) active site complexed with hCE (orange) is shown, with catalytic residues shown as green sticks and the hCE RCL harboring Asn39I colored in blue. The electron density map (2 Fobs−Fcalc) defining succinimide 147 (Suc147) is contoured at 1σ over the mean. c) Asp147 is essential for the religation reaction. The experiment described in (a) was repeated utilizing a combination of D147S AEP and hCE. The D147S mutant cleaved hCE, confirming its correct folding. By contrast, neither a shift in pH nor addition of MMTS led to resynthesis of hCE, demonstrating the critical role of Asp147 in the religation reaction. Analogous results were obtained with a D147G mutant.
