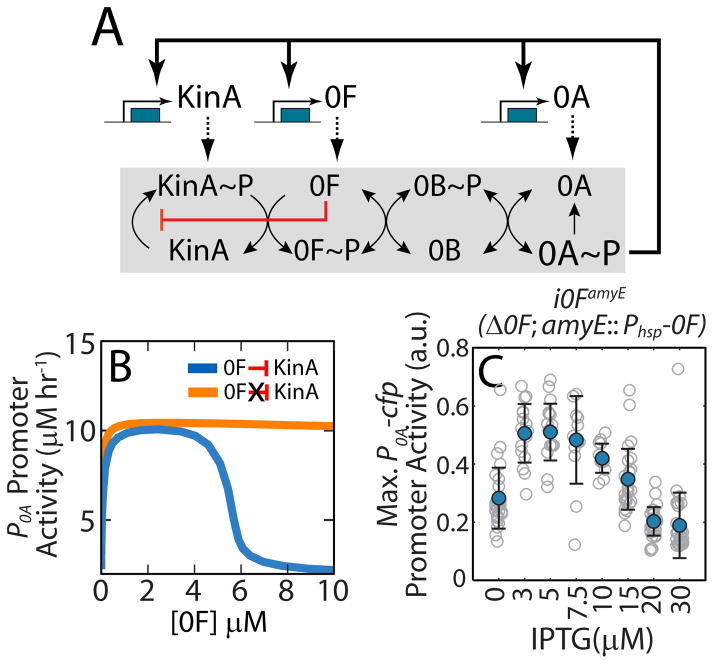
Figure 2.
Substrate inhibition of 0F by KinA produces a negative feedback in the phosphorelay.
A. Network diagram of the sporulation phosphorelay network that controls the activity of the master regulator Spo0A (0A). The phosphorelay includes both post-translational and transcriptional regulatory interactions. Post-translationally, the kinases KinA-E (only KinA is shown) transfer phosphoryl groups to the master regulator 0A via the two phosphotransferases Spo0B (0B) and Spo0F (0F). Transcriptionally, 0A~P controls the expression of kinA, 0F and 0A forming multiple transcriptional feedback loops. Our model also includes a substrate inhibition interaction (red blunted arrow) whereby excess 0F can bind to un-phosphorylated KinA and block its auto-phosphorylation. This substrate inhibition creates a negative feedback loop wherein 0A~P activates 0F expression and 0F inhibits 0A activation by inhibiting KinA. B. Mathematical model predicts that, for a phosphorelay with substrate-inhibition of KinA by 0F (blue curve), 0A promoter activity is a non-monotonic functions of 0F concentrations and decrease ultrasensitively for [0F]>5μM. In contrast, for a phosphorelay without substrate-inhibition (orange curve), 0A promoter activity monotonically increase to saturated value. C. Predicted non-monotonic dependence of 0A activity on 0F levels is confirmed by engineering inducible 0F strain, i0FamyE (Δ0F; amyE::Phsp-0F) and measuring maximum 0A promoter activity in the at different levels of 0F induction. Gray empty circles show maximum P0A promoter activity levels achieved by individual cell lineages over 25 hours in starvation conditions at each IPTG concentration. Blue filled circles and error-bars indicate the mean and standard deviations of these measurements at each IPTG concentration. Maximum P0A promoter activity decreases at high 0F expression levels (IPTG>10μM) in agreement with the substrate-inhibition effect of 0F overexpression.