Figure 3.
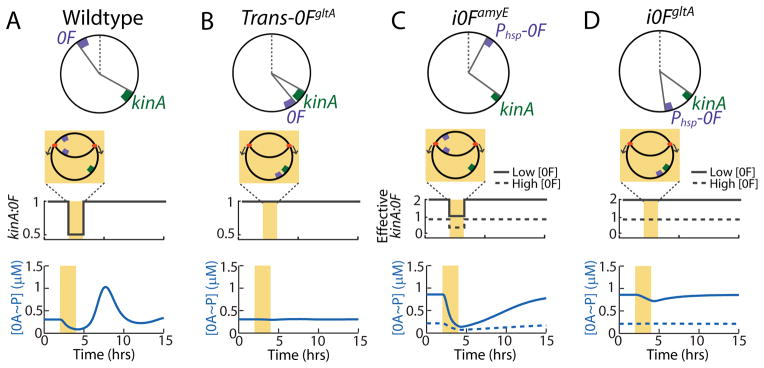
Mathematical model identifies the mechanism of 0A~P pulsing and its necessary conditions.
Top panels in (A–D) show chromosomal arrangements of 0F and kinA in (A) Wildtype (WT) B. subtilis and engineered strains: (B) Trans-0FgltA, (C) i0FamyE, (D) i0FgltA. 0F is located close to the origin of replication in WT and i0FamyE strains and close to the terminus in the Trans-0FgltA and i0FgltA. kinA is located close to the terminus in all strains. Note that 0F is expressed from the IPTG-inducible Phsp promoter, rather than the native 0A~P regulated P0F promoter in the inducible i0FamyE and i0FgltA strains. Middle panels in (A–D) show changes in kinA:0F gene dosage ratio in the WT and engineered strains. In the inducible i0FamyE and i0FgltA strains, the effective kinA:0F gene dosage depends on whether the level of 0F expression from the IPTG-inducible Phsp promoter is low (solid line) or high (dashed line). Bottom panels in (A–D) show model predictions for the response of 0A~P levels to the changes in kinA:0F ratio in WT and engineered strains. Model simulations show that the transient decrease in kinA:0F during DNA replication (yellow bar) in WT (A) inhibits the phosphorelay phosphate flux, thereby causing a decrease in 0A~P. Once DNA replication is complete, the phosphorelay produces an overshoot of 0A~P before returning to the steady state resulting in a 0A~P pulse. Model results also predict that that elimination of the transient decrease of kinA:0F in the Trans-0FgltA strain (B) abolishes 0A~P pulsing and instead the system stays at steady state. In the i0FamyE strain (C), the transient decrease in effective kinA:0F causes a decrease in 0A~P but 0A~P does not overshoot before returning to the steady state and there is no pulse. depends on the level of 0F expression. Simulations also show that elimination of the transient decrease of kinA:0F in the i0FgltA strain (D) abolishes 0A~P pulsing and instead the system stays at steady state. This 0A~P response level in i0F strains depends on whether 0F expression from the IPTG-inducible Phsp promoter is low (solid line) or high (dashed line). These results show that 0A~P pulsing is triggered by DNA replication and that both the kinA-0F chromosomal arrangement and 0A~P-0F negative feedback are essential for 0A~P pulsing.
