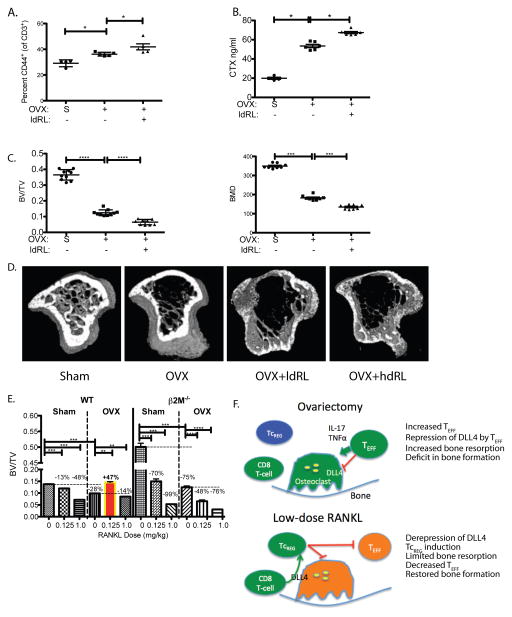
Figure 7. The anabolic effect of low-dose RANKL treatment in ovariectomized mice is mediated through CD8 T-cells.
To confirm that decrease in bone erosion and effector T-cell levels is mediated via TcREG, β2M−/− mice lacking CD8 T-cells, were sham-operated (S; n=6), ovariectomized (OVX; n=6) and ovariectomized then treated with low-dose RANKL (ldRL = 0.125 mg/kg; n=6). In some experiments mice are also treated with high-dose RANKL (hdRL = 1 mg/kg). A. Low-dose RANKL treatment of ovariectomized mice lacking CD8 T-cells does not decrease TEFF: Ovariectomy of β2M−/− mice increased effector T-cells (CD3+ and CD44+) relative to sham-operated (indicated by S). However, unlike treatment of CD8 T-cell-replete mice, no decrease in levels of effector T-cells was observed due to low-dose RANKL (ldRL) treatment in β2M−/− mice. B. Low-dose RANKL treatment of ovariectomized mice lacking CD8 T-cells does not decrease bone resorption: Bone resorption levels, as assayed by serum CTX, increase in CD8 T-cell (β2M−/−) deficient ovariectomized mice relative to sham-operated mice. A modest increase in serum CTX is observed in ovariectomized β2M−/− mice treated with low-dose RANKL. C. Low-dose RANKL treatment of ovariectomized mice lacking CD8 T-cells does not increase bone volume or bone density: Ovariectomy leads to significant levels of bone loss relative in β2M−/− mice. Low-dose RANKL leads to further decrease in bone volume (BV/TV) and bone mineral density (BMD) in β2M−/− mice, indicating that CD8 T-cells in WT mice protect against bone loss. D. Representative μCT renderings for panels C and E. E. Low-dose RANKL treatment of ovariectomized mice is bone anabolic but not in mice lacking CD8 T-cells: In WT mice low-dose RANKL is anti-resorptive in ovariectomized mice but promotes bone loss in estrogen-replete mice. Treatment with high-dose RANKL (1mg/kg; n= 6 mice per group) leads to bone resorption in both estrogen-deficient and replete mice. In CD8 T-cell-deficient mice low dose RANKL leads to bone loss in both estrogen-replete (Sham) and ovariectomized (OVX) mice. Percentage of bone volume lost (or gained; BV/TV) relative to reference state (untreated sham surgery or ovariectomy - indicated by horizontal dashed line) is also given. The results represent mean ± standard deviation (error bars). P values were calculated using Mann-Whitney test; *** = P < 0.001 and * = P < 0.05. F. A model of the negative feedback loop between osteoclast-induced TcREG: In ovariectomized mice increased levels of proinflammatory cytokines TNFα and IL-17 suppress DLL4 expression needed for TcREG induction (left panel). In the absence of TcREG and in the presence of the proinflammatory cytokine the activated osteoclasts have increased bone resorption leading to osteoporosis. Treatment with low-dose RANKL (right panel) reverses the repression of DLL4 in ovariectomized mice leading to induction of TcREG by osteoclasts to engage the negative feedback loop. Induction of TcREG not only limits osteoclastogenesis but also reduced the proinflammatory effector T-cells. The reduction in TEFF leads to increased bone formation rate.