Abstract
OBJECTIVES
The purpose of this study is to determine the trajectory of lung function change after exposure cessation to occupational organic dust exposure, and to identify factors that modify improvement.
METHODS
The Shanghai Textile Worker Study is a longitudinal study of 447 cotton workers exposed to endotoxin-containing dust and 472 silk workers exposed to non-endotoxin-containing dust. Spirometry was performed at 5 year intervals. Air sampling was performed to estimate individual cumulative exposures. The effect of work cessation on FEV1 was modeled using generalized additive mixed effects models to identify the trajectory of FEV1 recovery. Linear mixed effects models incorporating interaction terms were used to identify modifiers of FEV1 recovery. Loss to follow-up was accounted for with inverse probability of censoring weights.
RESULTS
74.2% of the original cohort still alive participated in 2011. Generalized additive mixed models identified a non-linear improvement in FEV1 for all workers after exposure cessation, with no plateau noted 25 years after retirement. Linear mixed effects models incorporating interaction terms identified prior endotoxin exposure (p=0.01) and male gender (p=0.002) as risk factors for impaired FEV1 improvement after exposure cessation. After adjusting for gender, smoking delayed the onset of FEV1 gain but did not affect the overall magnitude of change.
CONCLUSIONS
Lung function improvement after cessation of exposure to organic dust is sustained. Endotoxin exposure and male gender are risk factors for less FEV1 improvement.
Keywords: cotton, endotoxin, FEV1, recovery of function, occupational diseases, gender
INTRODUCTION
Chronic obstructive pulmonary disease (COPD) is projected to be the fourth leading cause of death worldwide.[1] Although tobacco smoke is most commonly identified as the main environmental exposure associated with COPD development, occupational exposures are thought to contribute 15% of the population attributable risk of COPD,[2] with estimates as high as 30% in non-smokers.[3] However, the vast majority of studies on occupational exposures focus on lung function changes during active exposure; few are available to describe lung function after exposure cessation due to worker retirement. Whether lung function recovers after removal from an occupational exposure is not well understood.
Exposure to endotoxin-containing cotton dust has been associated with the development of chronic lung disease. During early exposure, the disease is asthma-like, with airway hyper-reactivity and reversible airflow obstruction, while later disease resembles COPD, with fixed airflow obstruction and more prominently, an accelerated decline in forced expiratory volume in one second (FEV1). Autopsy series suggest airways disease and emphysema as the primary pathologic lesions.[4] Human studies have affirmed that it is the amount of endotoxin rather than the amount of dust in cotton exposure that determines both acute[5] and chronic[6] declines in FEV1. While endotoxin is a common occupational exposure,[7] it is also a common environmental exposure, and is present at high concentrations in urban school,[8] homes burning biomass fuel,[9] and tobacco smoke.[10]
The Shanghai Textile Worker Study is the longest active longitudinal study of cotton and silk textile workers. While cotton dust contains high levels of endotoxin, silk dust contains near-undetectable levels of endotoxin, creating a natural experiment in which to study the long term effects of exposure to endotoxin-containing organic dust. Our primary goal was to evaluate whether the transient FEV1 improvement noted after cessation of occupational dust exposure due to worker retirement in the 25-year follow-up of [11] this study was sustained given further follow-up and use of more flexible modelling techniques. Our secondary goal was to determine whether prior occupational endotoxin exposure, smoking, and gender modify FEV1 improvement.
METHODS
Study population and study design
919 workers from two cotton and one silk textile mill in the same industrial sector in Shanghai, China were recruited in 1981 (study schema in Supplemental Figure 1). The main inclusion criterion was at least two years of work in the identified mills in order to ensure a stable study population. The main exclusion criterion was a history of prior respiratory disease. The study population represented 90% of eligible workers in the yarn preparation areas of the three mills.[12] Cotton and silk workers were comparable in 1981 at the start of the study with respect to income, place of residence, and other socio-economic factors due to the hiring practices of the Shanghai Textile Bureau. Surveys were performed in 1981, 1986, 1992, 1996, 2001, 2006, and 2011, with eligibility for retesting based on presence in the baseline 1981 survey. Pre-bronchodilator spirometry, physical exam, modified American Thoracic Society symptom, work history, and smoking questionnaires, and exposure assessment (in the period prior to worker retirement) was performed at each survey. Forced expiratory maneuvers (up to seven trials to produce three acceptable curves) according to American Thoracic Society guidelines were performed under the direction of a trained technician on calibrated 8L water-sealed field spirometers (W E Collins, Braintree, Massachusetts, USA), and spirometric curves were manually read by the same trained expert. The highest values for forced expiratory volume in one second (FEV1) were used given that they were technically acceptable tests. A total of four spirometers, all of the same make and from the same manufacturer, were used for all field surveys from 1981 to 2011. Informed consent was obtained from all subjects and the study was approved by the Institutional Review Boards at the Harvard School of Public Health and the Shanghai Putuo District People's Hospital.
Exposure assessment
Exposure assessment was performed as previously described[13 14]. Multiple area samples were collected from each of the 6 different work areas in the two cotton mills using vertical elutriators to collect respirable fractions of cotton dust, with sampling times ranging from 3 to 7 hours. Filters were subsequently transported to a single laboratory at the National Institute of Occupational Safety and Health for endotoxin analysis. Endotoxin from collected filters was measured using a Limulus amebocyte lysate gel test (Pyrostat-50), and values for each filter were summed and converted from ng/ml to μg/m3 based on sampling time and air flow rates of each sampler. Exposure measurements collected in the first survey were used to estimate pre-1981 samples. 6 full-shift samples in the silk mills had near-undetectable levels of endotoxin (0.001 EU/m3) in vertical elutriator samples; thus silk workers were considered unexposed to occupational endotoxin. Individual endotoxin exposure was calculated using geometric means of endotoxin measured in each work area multiplied by years of work in each work area, resulting in a lifetime cumulative index of occupational exposure measured in endotoxin units/meters3-years (EU/m3-yrs), with an interpretation analogous to that of pack-years for smoking. At each survey, a detailed work history was obtained to identify the date of textile work cessation as well as job descriptions post retirement.
Statistical Analysis
The primary outcome of interest was change in FEV1 associated with work cessation. However, in order to adjust for the potential bias from loss to follow-up using inverse probability of censoring weights, in our statistical models, FEV1 rather than change in FEV1 was used as the primary outcome measure. Covariates for the outcome models included age, gender, height, smoking status (defined as lifetime never, current or former), and cumulative pack-years. Exposure was modelled as either cotton vs. silk textile work, or as log-transformed measured cumulative occupational endotoxin exposure.
We modelled FEV1 using a generalized additive mixed effects model (GAMM)[15] with a penalized spline term for the number of years since work cessation. Such use of a GAMM allows the data to identify the functional form of the relationship between exposure cessation and FEV1 change, rather than constraining the relationship based on modeling decisions. Our secondary research question focused on whether lung function recovery was modified by prior occupational endotoxin exposure, smoking, or gender. The significance of an interaction between a categorical variable (i.e. cotton vs. silk) and a smoothed term (penalized spline term for work cessation-years) cannot be estimated in a generalized additive mixed model. Therefore the final outcome model was a linear mixed model with both linear and quadratic terms for work cessation as suggested by the GAMM (see Supplement for details).
As mentioned, FEV1 rather than change in FEV1 was used as the outcome measure in our statistical models. Therefore the main effect of group represents baseline differences in FEV1, whereas a group*time interaction represents the change in FEV1 associated with that grouping variable in a longitudinal study.[16] Interaction terms between work cessation years and occupational exposure, smoking, and gender were included in all models in order to determine whether changes in FEV1 were modified by these variables. Models with random intercept and slope to account for within subject correlation over time were used.
Despite the high rate of participation at our 30 year survey, it is possible that loss to follow-up may lead to bias if missing data is not accounted for. For observations with a monotone pattern of missingness (ie the subject never participated in another survey after the first missed survey), it was assumed that the missing data mechanism was missing at random (MAR). This mechanism implies that missingness can be explained by observed variables such as older age, presence of respiratory symptoms, or occupational exposure. To adjust for the possibility that loss to follow-up differed by case history, stabilized inverse probability of censoring weights[17] were used in the final models. The denominator of the weights was based on a logistic model predicting that the outcome was uncensored, i.e. a technically acceptable FEV1 measurement was present. Predictors were cotton vs. silk exposure, age, gender, work cessation-years, years worked in the textile industry, and both presence of respiratory symptoms and FEV1 at the preceding survey. The numerator of the weights was based on a logistic model for the same outcome, but included only exposure (cotton vs. silk work) as the predictor.
Percent predicted FEV1 was calculated based on prediction equations derived from Chinese populations.[18] Statistical analyses were performed using R 3.1.0 with the packages lme4,[19] mgcv,[15] and ipw.[20]
RESULTS
919 workers (447 cotton and 472 control silk workers) were recruited in 1981 to participate in the Shanghai Textile Worker Study (Supplemental Figure 1). The median number of FEV1 measurements obtained was 6 [interquartile range 4-7] per subject. The cotton and silk textile workers were overall quite comparable in both 1981 and 2011 (Table 1) although a higher proportion of cotton workers smoked compared to silk workers in 1981. Very few females smoked (one silk, ten cotton workers in 1981; one silk, three cotton workers in 2011). In 1981, when all of the workers were actively working, cotton textile workers had more respiratory symptoms compared to silk textile workers. At the most recent survey in 2011, there were no significant differences in the proportion of cotton vs. silk workers with respiratory symptoms. In 2011, the average duration of retirement was 18 years for both cotton and silk workers. Most of the textile workers retired between 1992 and 2001, with only three (two silk, one cotton) still active in textile work in 2011. There were no significant differences in follow-up rates between cotton and silk workers, with a similar average duration of follow-up in cotton compared to silk workers. Lifetime cumulative occupational endotoxin exposure was on average 38,928 [interquartile range 17,30-65,204] EU/m3-years for cotton workers in 2011, whereas it was assumed to be negligible for silk workers based on a limited number of full shift samples taken in silk mills which demonstrated near undetectable levels of endotoxin.
Table 1.
Characteristics of study participants at baseline (1981) and end of follow-up (2011).
| 1981 | 2011 | |||
|---|---|---|---|---|
| Silk (n=472) | Cotton (n=447) | Silk (n=291) | Cotton (n=296) | |
| Male | 200 (42.4%) | 214 (47.9%) | 112 (38.5%) | 122 (41.2%)* |
| Age, years | 36.7 ± 10.7 | 37.8 ± 10.6 | 65.8 ± 9.7 | 65.6 ± 9.9 |
| Follow-up time, years | - | - | 29.4 ± 0.07 | 29.6 ± 0.06 |
| Height, cm | 162.5 ± 7.3 | 163.9 ± 7.5* | 160.6 ± 7.6 | 162.1 ± 7.9 |
| Current smoking | 118 (25.0%) | 159 (35.6%)* | 60 (20.6%) | 66 (22.3%) |
| Male | 117 (58.5% of males) | 149 (69.6% of males) | 59 (52.7% of males) | 63 (51.6% of males) |
| Female | 1 (0.9% of females) | 10 (2.2% of females) | 1 (0.6% of females) | 3 (1.7% of females) |
| Pack-years a | 9.2 ± 10.0 | 8.5 ± 9.8 | 28.2 ± 19.8) | 28.2 ± 19.0 |
| Lifetime nonsmoker | 348 (73.7%) | 284 (65.5%)* | 182 (66.9%) | 178 (62.9%) |
| Male | 77 (38.5% of males) | 61 (28.5% of males) | 22 (20.2% of males) | 25 (20.8% of males) |
| Female | 271 (99.6% of females) | 223 (95.7% of females) | 160 (98.2% of females) | 153 (93.9% of females) |
| Active textile work | 472 (100%) | 447 (100%) | 2 (0.34%) | 1 (0.17%) |
| Years employed | 16.2 ± 11.3 | 16.2 ± 10.3 | 27.3 ± 9.2 | 26.3 ± 8.0 |
| Years since retirement b | - | - | 18.5 ± 4.5 | 17.8 ± 5.2 |
| Cumulative endotoxin exposure, EU/m3-yrsc | - | 11,540 [3,442-32,111] | - | 38,928 [17,030- 65,204] |
| FEV1, liters | 2.84 ± 0.67 | 2.90 ± 0.72 | 2.29 ± 0.58 | 2.28 ± 0.64 |
| % predicted FEV1 d | 99.6 ± 13.1 | 100.2 ± 13.9 | 107.7 ± 18.9 | 104.4 ± 18.9 |
| FEV1/FVC ratio | 0.84 ± 0.09 | 0.83 ± 0.09 | 0.77 ± 0.08 | 0.76 ± 0.08 |
| FEV1/FVC<0.7 e | 20 (4.2%) | 27 (6.0%) | 39 (13.4%) | 40 (13.5%) |
| FEV1 ≥80% predicted | 12 | 16 | 31 | 30 |
| 50% ≤ FEV1 <80% predicted | 7 | 10 | 7 | 7 |
| 30% ≤ FEV1 <50% predicted | 1 | 1 | 1 | 2 |
| FEV1 <30% predicted | 0 | 0 | 0 | 1 |
| Unadjusted annual FEV1 change (mL/yr) | - | - | −19.6 ± 12.1 | −23.0 ± 12.9* |
| Chronic bronchitis | 36 (7.6%) | 96 (21.5%)* | 22 (2.6%) | 29 (4.9%) |
| Chronic cough | 33 (7.0%) | 87 (19.5%)* | 7 (1.2%) | 11 (1.9%) |
| Dyspnea on exertion | 18 (3.8%) | 67 (15.0%)* | 71 (12.1%) | 67 (11.4%) |
| Byssinosis | 0 (0%) | 34 (7.6%)* | 0 (0%) | 0 (0%) |
p±0.05. Continuous variables are presented as mean ± standard deviation or median [interquartile range]. Categorical variables are presented as n (%).
Calculated among ever-smokers only.
Calculated among retired workers only.
A limited number of full shift samples taken in silk mills were found to have undetectable levels of endotoxin by the limulus amebocyte lysate assay. Thus silk workers were considered unexposed to endotoxin.
prediction equations derived from Chinese reference population[18]
pre-bronchodilator spirometry
Individual unadjusted FEV1 and percent predicted FEV1 trajectories with age are depicted in Supplemental Figures 2 and 3. FEV1 trajectories differ between each strata of smoking and occupational exposure, with cotton smokers having the steepest decline over time.
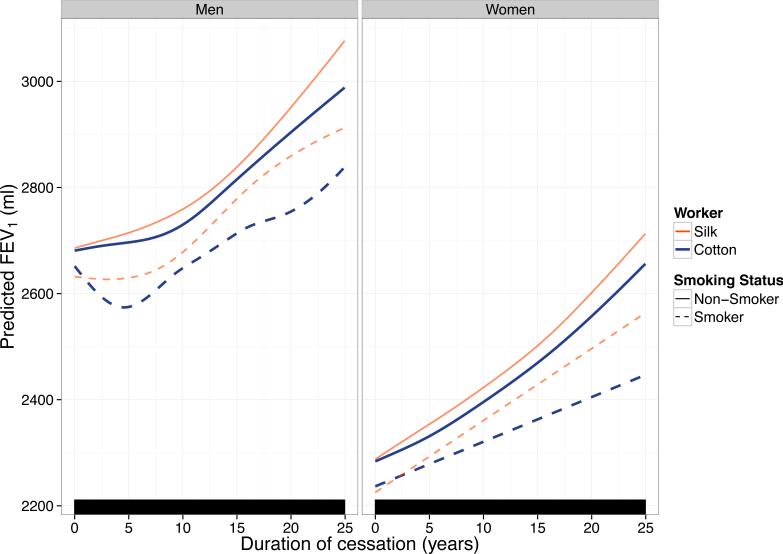
The adjusted effect of work cessation-years on FEV1 based on the GAMM model is depicted in Figure 1 and Table 2. Several observations can be made from these predictions. First, the effect of work cessation on FEV1 is non-linear, with no plateau in FEV1 improvement noted up to 25 years after work cessation in all strata. Second, the greatest improvements in FEV1 after work cessation are seen in non-smoking silk > non-smoking cotton > smoking silk > smoking cotton textile workers over the observation period for both men and women. Third, for smokers, a gain in FEV1 with work cessation as compared to active textile work was not seen immediately at the time of work cessation. In males, at 5 years of work cessation, a gain in FEV1 for non-smokers was 28.7 [−14.5, 71.9] mL for silk and 15.6 [−28.3, 59.4] mL for cotton workers, whereas for smokers there was no gain in FEV1 with average predicted changes being −2.3 [−35.5, 30.9] mL for silk and −76.8 [−113.4, −40.2] mL for cotton smokers (Table 2). Given the low number of female smokers in our cohort, we cannot rule out that the same phenomenon may occur in women as well.
Figure 1. Adjusted effect of work cessation on FEV1, stratified by gender, occupational exposure, and smoking status based on a generalized additive mixed model.
Predictions are for eight hypothetical workers with different gender, smoking, and occupational (cotton vs. silk) exposures. Predictions assume that these workers have the same age, height, pack-year history (zero if non-smokers, the average number of pack-years if smokers) at each value of work cessation-years. Rug plot (bottom) indicates values of cessation-years for which an observation was present. No plateau is seen in FEV1 improvement after work cessation. For both men and women, FEV1 improvement is greatest in non-smoking silk > non-smoking cotton > smoking silk > smoking cotton workers.
Table 2.
Predicted effect of work cessation on FEV1 by gender, occupational exposure, and smoking status based on a generalized additive mixed model.
| Change in FEV1, mL [95% CI] | ||||||||
|---|---|---|---|---|---|---|---|---|
| Men | ||||||||
| Cessation years | Silk non-smokers | Silk smokers | Cotton non-smokers | Cotton smokers | ||||
| 5 | 28.7 | [−14.5, 71.9] | −2.3 | [−35.5, 30.9] | 15.6 | [−28.3, 59.4] | −76.8 | [−113.4, −40.2] |
| 10 | 73.2 | [11.8, 134.6] | 46.2 | [4.2, 88.3] | 49.0 | [−11.8, 109.8] | −3.7 | [−48.4, 41.0] |
| 15 | 152.7 | [74.8, 230.6] | 146.8 | [95.7, 197.8] | 134.4 | [59.1, 209.7] | 61.9 | [7.1, 116.7] |
| 20 | 265.2 | [154.1, 376.2] | 227.3 | [155.1, 299.6] | 223.2 | [124.3, 322.2] | 103.0 | [26.1, 179.8] |
| 25 | 391.8 | [214.2, 569.4] | 280.8 | [161.3, 400.4] | 308.0 | [159.5, 456.6] | 187.8 | [76.8, 298.7] |
| Women | ||||||||
| Cessation years | Silk non-smokers | Silk smokers | Cotton non-smokers | Cotton smokers | ||||
| 5 | 66.1 | [43.8, 88.4] | 67.8 | [−21.8, 157.3] | 47.4 | [24.4, 70.4] | 42.1 | [−1.0, 85.1] |
| 10 | 135.2 | [103.8, 166.7] | 135.5 | [−43.6, 314.6] | 112.0 | [79.6, 144.3] | 84.1 | [−1.9, 170.2] |
| 15 | 213.9 | [175.0, 252.8] | 203.3 | [−65.4, 472.0] | 185.7 | [145.5, 226.0] | 126.2 | [−2.9, 255.3] |
| 20 | 313.9 | [264.1, 363.8] | 271.0 | [−87.2, 629.3] | 273.7 | [221.3, 326.0] | 168.3 | [−3.8, 340.4] |
| 25 | 426.1 | [358.7, 493.4] | 338.8 | [−109, 786.6] | 373.2 | [302.3, 444.2] | 210.4 | [−4.8, 425.5] |
Predictions are for eight hypothetical workers with different gender, smoking, and occupational (cotton vs. silk) exposures. Predictions assume that these workers have the same age, height, pack-year history (zero if non-smokers, the average number of pack-years if smokers) at each value of work cessation-years. Values represent the adjusted change in FEV1 [95% CI], in mL, for that value of work cessation-year, as compared to active work.
To determine whether occupational endotoxin exposure, smoking, or gender modifies FEV1 recovery after work cessation, interaction terms were added to the mixed effects models (Table 3). Ten years of work cessation was associated with an average 168.5 mL improvement in FEV1 in all textile workers. Cotton workers had 25.8 mL less improvement compared to silk workers (p=0.02), and men had 4.9 mL (p=0.003) less improvement compared to women. Current smokers had 15.4 mL less improvement compared to non-smokers (p=0.63) although the relationship was not statistically significant. Although few female textile workers were smokers, the effect of gender was not due solely to the effect of smoking. When the analysis was restricted to lifetime non-smokers, the gender*cessation interaction term remained statistically significant (p-value = 0.002).
Table 3.
Factors modifying FEV1 change (in mL) after work cessation based on a linear mixed effects model, with exposure modelled as cotton vs. silk.
| FEV1, mL [95% CI] |
||||
|---|---|---|---|---|
| All | Non-smokers | |||
| Cessation-years | 14.2*** | [12.0, 16.5] | 9.4*** | [7.1, 11.7] |
| Cessation-years2 | 0.3*** | [0.1, 0.4] | 0.3*** | [0.2, 0.5] |
| Cotton*cessation-years | −2.7* | [−5.0, −0.5] | −1.7a | [−4.2, 0.7] |
| Current smoker*cessation-years | 1.1 | [−2.6, 4.9] | - | - |
| Former smoker*cessation-years | 0.4 | [−4.4, 5.2] | - | - |
| Male*cessation-years | −5.7** | [−9.1, −2.3] | −4.5** | [−7.8, −1.3] |
| Observations | 4,702 | 3,068 | ||
p<0.05
p<0.01
p<0.001
Note: Quadratic interaction terms were significant only for the male*cessation-years2 term, with β=0.5 [0.2, 0.8], p=0.0009 for all workers and β=0.4 [0.1, 0.7], p=0.02 for the analysis restricted to non-smokers.
cotton*cessation-years interaction p=0.08 in analysis restricted to non-smokers
Model adjusted for age, height, gender, exposure (cotton vs. silk), smoking (never, current, former), cumulative pack-years, and interaction terms between cessation-years and occupational exposure, cessation-years and smoking, and cessation-years and gender. Loss-to follow-up accounted for using inverse probability of censoring weights. Note: as the modelled outcome was FEV1 and not change in FEV1, the interaction terms with cessation-years represent the associations between occupational exposure, smoking, gender, and change in FEV1 after leaving work.
When occupational exposure was modelled as a log-transformed measure of cumulative endotoxin exposure (Table 4), the interaction between work cessation-years and endotoxin remained significant (p=0.01), indicating a dose-dependent relationship between prior occupational endotoxin exposure and less FEV1 improvement.
Table 4.
Factors modifying FEV1 change (in mL) after work cessation based on a linear mixed effects model, with exposure modelled as cumulative occupational endotoxin exposure.
| FEV1, mL [95% CI] | ||||
|---|---|---|---|---|
| All | Non-smokers | |||
| Cessation-years | 11.8*** | [9.7, 13.9] | 7.9*** | [5.7, 10.1] |
| Cessation-years2 | 0.3*** | [0.1, 0.4] | 0.3*** | [0.2, 0.5] |
| Endotoxin*cessation-years | −0.1* | [−0.2, −0.02] | −0.1a | [−0.1, 0.03] |
| Current smoker*cessation-years | 1.1 | [−2.6, 4.9] | - | - |
| Former smoker*cessation-years | 0.4 | [−4.4, 5.2] | - | - |
| Male*cessation-years | −5.7** | [−9.1, −2.3] | −4.5** | [−7.8, −1.3] |
| Observations | 4,702 | 3,068 | ||
p<0.05
p<0.01
p<0.001
Note: Quadratic interaction terms were significant only for the male*cessation-years2 term, with β=0.5 [0.2, 0.8], p=0.001 for all workers and β=0.4 [0.1, 0.7], p=0.02 for the analysis restricted to non-smokers.
cotton*cessation-years interaction p=0.08 in analysis restricted to non-smokers.
Model adjusted for age, height, gender, exposure (cotton vs. silk), smoking (never, current, former), cumulative pack-years, and interaction terms between cessation-years and occupational exposure, cessation-years and smoking, and cessation-years and gender. Loss-to follow-up accounted for using inverse probability of censoring weights. Note: as the modelled outcome was FEV1 and not change in FEV1, the interaction terms with cessation-years represent the associations between occupational exposure, smoking, gender, and change in FEV1 after leaving work.
DISCUSSION
In this report, we demonstrate that retirement from work and therefore cessation of exposure to organic dust results in a sustained improvement in FEV1. The effect of exposure cessation, however, had a complex, non-linear relationship with FEV1. Importantly, recovery was adversely modified by prior occupational endotoxin exposure and male gender. After adjusting for gender, smoking was not associated with a statistically significant impact on FEV1 change after work cessation, although it appeared to impact the trajectory of improvement. To our knowledge, this is the first report to address whether lung function recovery is transient or sustained after occupational organic dust exposure, and also the first to identify prior occupational endotoxin exposure and gender as risk factors for decreased FEV1 recovery.
Whether FEV1 improves after exposure cessation in workers exposed to endotoxin-containing organic dust has been controversial. In the earliest longitudinal study to evaluate the effect of retirement, retired hemp workers had more respiratory symptoms and greater (but not statistically significant) annual declines in FEV1 (53.3 vs. 47.1 mL/year) at 9 year follow-up.[21] A subsequent 6-year study of cotton textile workers also found more respiratory symptoms and greater annual decline in FEV1 in retired compared to active workers.[22] Studies in retired grain elevator workers, another group with occupational exposure to endotoxin-containing organic dust, found no improvement with retirement.[23] One study looking at removal from exposure to endotoxin in a bacterial single cell protein factory found that FEV1 improved by 210 mL one year after exposure cessation in workers exposed to low levels of endotoxin, but no improvement in those exposed to high endotoxin levels.[24] Our own early studies on cessation did not find a significant association between work cessation and FEV1 improvement,[6] and it was only at 25-year follow-up[11] that we first reported FEV1 improvement after cessation of textile work, although we reported that improvement plateaued, with a trend towards greatest improvement in smoking cotton workers. In the present analysis, we clarify that improvement is sustained, male cotton workers improve the least over time, and further identify prior occupational exposure and gender as important modifiers of FEV1 recovery. There are several major differences between this and our prior work that may explain the apparent inconsistencies. First, we have an additional 5 years of follow-up. Second, our prior analysis did not allow for complex non-linearity. Third, we previously restricted our analysis to only subjects who participated at every survey since 1981; here we used all available data from all subjects while adjusting for loss to follow-up. Of particular interest is the observed lag between exposure cessation and an overall gain in FEV1 in smokers. This delayed recovery may provide an explanation for the negative results of studies with less than 10 years of follow-up, where both smokers and men comprise a significant proportion of the population studied, and highlights the importance of both long-term follow-up and use of advanced regression techniques when studying the recovery of lung function after exposure to a toxic environmental exposure.
The mechanism of lung function improvement after occupational dust exposure is unclear. Animal studies of repeated endotoxin exposure suggest that there are exposure-related structural changes such as epithelial and mesenchymal fibroproliferation[25] along with emphysema.[26] This implies that some component of endotoxin-related chronic lung disease is irreversible. Other studies demonstrate that repeated endotoxin exposure is associated with an expansion in the pro-inflammatory dendritic cell subsets in the lung.[27] It is possible that with exposure cessation, there is slow resolution of the inflammatory process. In preliminary studies, we have noted persistent changes in lung density on high resolution chest imaging in cotton workers that may represent ongoing inflammation decades after exposure cessation[28]. Future biomarker studies to identify the underlying basis for persistent effects of occupational endotoxin exposure decades after exposure cessation may be informative.
Furthermore, it is not clear why there are gender differences in recovery. A meta-analysis of person-level data pooled from 12 cross-sectional studies of workers exposed to organic dust found that women were less likely than men to experience lower respiratory symptoms within the same industry, although this study did not include exposure assessment and so was unable to exclude differences in gender specific workplace exposures as the explanation.[29] We have previously reported in our cohort that endotoxin exposed men are at higher risk than women of developing reduced lung function and mortality due to all causes of death combined.[30] Animal studies demonstrating an augmented response to endotoxin related to male sex hormones[31] provide a potential biological explanation. However, gender differences in FEV1 recovery were also noted among silk workers. Job descriptions after retirement were manually reviewed to determine whether workers remained in an environment with high endotoxin exposure; however, beyond job descriptions, further exposure assessment was not performed. Additional gender specific differences in exposure may have existed after worker retirement, or alternatively, gender may have a biological effect on FEV1 recovery.
Our study has several strengths. First, most studies on the effect of exposure cessation are cross-sectional with matched population controls, or are longitudinal studies of shorter duration. Our study spans 30 years, with little loss to follow-up, high participation, and large number of FEV1 measurements per subject. Second, while a long duration of follow-up is desirable, an improvement in lung function over time might be attributed to a survivor bias if loss to follow-up was not accounted for. Third, exposure assessment was performed[13 14] rather than reliance on a surrogate such as number of years worked. Finally, a large number of non-smoking men participated, allowing us to demonstrate that gender has effects on FEV1 recovery independent from smoking.
We acknowledge limitations to our study. First, although control silk workers were not exposed to endotoxin in the workplace, they were exposed to other organic dust and this likely explains why there was FEV1 recovery after leaving the workplace. Others have demonstrated that silk dust can have adverse respiratory effects.[32] However, measured endotoxin and dust levels in the cotton mills were not well correlated (correlation 0.38), and endotoxin levels in the cotton mills were significantly higher than in silk mills (836 vs. 0.001 EU/m3); thus it is unlikely that the exposure response relationship we observed with endotoxin is spurious. While the effect of occupational endotoxin exposure on recovery may appear small, the magnitude is comparable to that observed for tobacco smoke in our study. Second, there may be limited generalizability. Our study population is composed entirely of Han Chinese subjects; population differences in the prevalence of genetic polymorphisms known to confer differences in risk[33] from endotoxin may exist. Common in occupational studies is the presence of the healthy worker survivor effect, which may be more prominent in cotton textile workers.[34 35] We selected for cotton and silk workers who were free of respiratory disease after two years of work in the textile workforce; thus, the most susceptible workers were probably excluded from this study and may explain why few subjects ultimately met criteria for COPD. It is likely that in a less healthy population, the effect of endotoxin exposure on lung function would be larger than that seen in our study. While exposure misclassification is possible as we used area samplers in combination with job histories to estimate individual exposures, misclassification would be expected to be non-differential, and use of area samplers to estimate individual endotoxin exposure has been shown to be a reasonable surrogate.[36] Finally, while we used endotoxin to estimate exposure to gram-negative bacteria, it is clear that this surrogate of microbial load does not capture the complexity of exposures present in organic dust. Cotton dust contains a diverse variety of bacteria[37] as well as fungi[38]. Recent studies of environmental microbial exposures using high-throughput sequencing to identify microbial type have shown that it may be the presence of specific microbes,[39] or the diversity of microbial exposure,[40] that determines whether the ultimate effect on health is protective or harmful. We do not have residual organic dust to further refine the specific microbial exposures (of which endotoxin may be a marker of) that is associated with decreased lung function recovery. The identification of specific microbes that are harmful using sequencing techniques may represent exciting future areas of research.
In summary, this study is the first to demonstrate that FEV1 improvement is sustained long after cessation of workplace exposure to organic textile dust. However, the FEV1 improvement with exposure cessation is delayed in some subgroups, suggesting that studies of recovery of lung function after cessation of an environmental exposure needs to be of a sufficiently long duration. While lung function recovery is sustained, male gender adversely affects recovery, suggesting that men may represent a sub-population that would benefit from early screening for respiratory disease. Despite exposure cessation, past exposure to occupational endotoxin has an exposure-dependent relationship with decreased FEV1 recovery, affirming the importance of workplace limits on not just the amount of organic dust, but also the amount of endotoxin.
Supplementary Material
WHAT THIS PAPER ADDS
Long term exposure to organic dust is associated with an accelerated decline in lung function.
Few longitudinal studies are available to describe whether there is sustained improvement in lung function after exposure cessation to occupational organic dust. Whether there are factors that modify this improvement is unknown.
This paper demonstrates that lung function continues to improve decades after work-related organic dust exposure. Men and those exposed to endotoxin improve less. Smoking delays the onset of lung function recovery but is not associated with the overall magnitude of recovery.
ACKNOWLEDGEMENTS
We wish to thank Dr. He-lian Dai for her contributions to establishing this cohort, and Marcia Chertok for manual review of spirometry tracings.
FUNDING
Funding was obtained from the National Institute of Occupational Safety and Health (NIOSH OH002421) and the National Institute of Health (NIH-NIEHS K23ES023700, F32ES020082, and ES00002). The study sponsors had no role in the study design, gathering, analysis, and interpretation of data, or in manuscript writing.
Footnotes
AUTHORS AND CONTRIBUTORS
PL, LV, EE, and DC contributed to the conceptual design of this manuscript. PL, JH, FZ, BZ, LS, AA, JS, and DC participated in the study implementation and data acquisition. DC established the cohort on which this study is based, and JH is the Shanghai local site investigator. PL, LV, DB, EE, DC participated in data analysis and interpretation of the results. PL wrote the manuscript with input from all authors.
COMPETING INTERESTS
The authors have no conflicts of interest to disclose.
REFERENCES
- 1.Mathers CD, Loncar D. Projections of global mortality and burden of disease from 2002 to 2030. PLoS medicine. 2006;3(11):e442. doi: 10.1371/journal.pmed.0030442. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Trupin L, Earnest G, San Pedro M, et al. The occupational burden of chronic obstructive pulmonary disease. Eur Respir J. 2003;22(3):462–9. doi: 10.1183/09031936.03.00094203. [DOI] [PubMed] [Google Scholar]
- 3.Mak GK, Gould MK, Kuschner WG. Occupational inhalant exposure and respiratory disorders among never-smokers referred to a hospital pulmonary function laboratory. Am J Med Sci. 2001;322(3):121–6. doi: 10.1097/00000441-200109000-00002. [DOI] [PubMed] [Google Scholar]
- 4.Lai PS, Christiani DC. Long-term respiratory health effects in textile workers. Current opinion in pulmonary medicine. 2013;19(2):152–7. doi: 10.1097/MCP.0b013e32835cee9a. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Castellan R, Olenchock S, Kinsley K, Hankinson J. Inhaled endotoxin and decreased spirometric values. New England Journal of Medicine. 1987;317(10):605–10. doi: 10.1056/NEJM198709033171005. [DOI] [PubMed] [Google Scholar]
- 6.Wang X-R, Zhang H-X, Sun B-X, et al. A 20-year follow-up study on chronic respiratory effects of exposure to cotton dust. Eur Respir J. 2005;26(5):881–6. doi: 10.1183/09031936.05.00125604. [DOI] [PubMed] [Google Scholar]
- 7.Liebers V, Raulf-Heimsoth M, Brüning T. Health effects due to endotoxin inhalation (review). Arch Toxicol. 2008;82(4):203–10. doi: 10.1007/s00204-008-0290-1. [DOI] [PubMed] [Google Scholar]
- 8.Jacobs JH, Krop EJ, de Wind S, Spithoven J, Heederik DJ. Endotoxin levels in homes and classrooms of Dutch school children and respiratory health. Eur Respir J. 2013;42(2):314–22. doi: 10.1183/09031936.00084612. [DOI] [PubMed] [Google Scholar]
- 9.Semple S, Devakumar D, Fullerton DG, et al. Airborne endotoxin concentrations in homes burning biomass fuel. Environ Health Perspect. 2010;118(7):988–91. doi: 10.1289/ehp.0901605. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Hasday JD, Bascom R, Costa JJ, Fitzgerald T, Dubin W. Bacterial endotoxin is an active component of cigarette smoke. Chest. 1999;115(3):829–35. doi: 10.1378/chest.115.3.829. [DOI] [PubMed] [Google Scholar]
- 11.Shi J, Hang JQ, Mehta AJ, et al. Long-term effects of work cessation on respiratory health of textile workers: a 25-year follow-up study. Am J Respir Crit Care Med. 2010;182(2):200–6. doi: 10.1164/rccm.200903-0329OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Christiani D, Ye T, Wegman D, Eisen E, Dai H, Lu P. Cotton dust exposure, across-shift drop in FEV1, and five-year change in lung function. Am J Respir Crit Care Med. 1994;150(5 Pt 1):1250–5. doi: 10.1164/ajrccm.150.5.7952548. [DOI] [PubMed] [Google Scholar]
- 13.Kennedy S, Christiani D, Eisen E, et al. Cotton dust and endotoxin exposure-response relationships in cotton textile workers. Am Rev Respir Dis. 1987;135(1):194–200. doi: 10.1164/arrd.1987.135.1.194. [DOI] [PubMed] [Google Scholar]
- 14.Olenchock SA, Christiani DC, Mull JC, Ye TT, Lu PL. Airborne endotoxin concentrations in various work areas within two cotton textile mills in the People's Republic of China. Biomedical and environmental sciences : BES. 1990;3(4):443–51. [PubMed] [Google Scholar]
- 15.Wood SN. Fast stable restricted maximum likelihood and marginal likelihood estimation of semiparametric generalized linear models. Journal of the Royal Statistical Society. 2011;73(1):3–36. [Google Scholar]
- 16.Fitzmaurice GM, Laird NM, Ware JH. Applied Longitudinal Analysis. Second Edition John Wiley & Sons; Hoboken, New Jersey: 2011. [Google Scholar]
- 17.Cole SR, Hernan MA. Constructing inverse probability weights for marginal structural models. Am J Epidemiol. 2008;168(6):656–64. doi: 10.1093/aje/kwn164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Ip MS, Ko FW, Lau AC, et al. Updated spirometric reference values for adult Chinese in Hong Kong and implications on clinical utilization. Chest. 2006;129(2):384–92. doi: 10.1378/chest.129.2.384. [DOI] [PubMed] [Google Scholar]
- 19.Bates D, Maechler M, Bolker B, Walker S. lme4: Linear mixed-effects models using Eigen and S4. R package version 1.1-6. 2014 http://CRAN.R-project.org/package=lme4.
- 20.van der Wal WM, Geskus RB. ipw: An R Package for Inverse Probability Weighting. Journal of Statistical Software. 2011;43(13):1–23. [Google Scholar]
- 21.Bouhuys A, Zuskin E. Chronic respiratory disease in hemp workers. A follow-up study, 1967-1974. Ann Intern Med. 1976;84(4):398–405. doi: 10.7326/0003-4819-84-4-398. [DOI] [PubMed] [Google Scholar]
- 22.Beck G, Schachter E, L M, Schilling R. A prospective study of chronic lung disease in cotton textile workers. Annals of Internal Medicine. 1982;97(5):645. doi: 10.7326/0003-4819-97-5-645. [DOI] [PubMed] [Google Scholar]
- 23.Kennedy SM, Dimich-Ward H, Desjardins A, Kassam A, Vedal S, Chan-Yeung M. Respiratory health among retired grain elevator workers. Am J Respir Crit Care Med. 1994;150(1):59–65. doi: 10.1164/ajrccm.150.1.8025773. [DOI] [PubMed] [Google Scholar]
- 24.Skogstad M, Sikkeland LI, Ovstebo R, et al. Long-term occupational outcomes of endotoxin exposure and the effect of exposure cessation. Occup Environ Med. 2012;69(2):107–12. doi: 10.1136/oem.2010.062414. [DOI] [PubMed] [Google Scholar]
- 25.Brass DM, Savov JD, Gavett SH, Haykal-Coates N, Schwartz DA. Subchronic endotoxin inhalation causes persistent airway disease. Am J Physiol Lung Cell Mol Physiol. 2003;285(3):L755–61. doi: 10.1152/ajplung.00001.2003. [DOI] [PubMed] [Google Scholar]
- 26.Brass DM, Hollingsworth JW, Cinque M, et al. Chronic LPS inhalation causes emphysema-like changes in mouse lung that are associated with apoptosis. Am J Respir Cell Mol Biol. 2008;39(5):584–90. doi: 10.1165/rcmb.2007-0448OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Lai PS, Fresco JM, Pinilla MA, et al. Chronic endotoxin exposure produces airflow obstruction and lung dendritic cell expansion. Am J Respir Cell Mol Biol. 2012;47(2):209–17. doi: 10.1165/rcmb.2011-0447OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Lai P, Hang J, Zhang F, et al. Cotton textile work is associated with persistent changes in lung density on quantitative CT scans. Am J Respir Crit Care Med. 2014;189:A5104. 2014. [Google Scholar]
- 29.Schachter EN, Zuskin E, Moshier EL, et al. Gender and respiratory findings in workers occupationally exposed to organic aerosols: a meta analysis of 12 cross-sectional studies. Environmental health : a global access science source. 2009;8:1. doi: 10.1186/1476-069X-8-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Lai PS, Hang JQ, Zhang FY, et al. Gender differences in the effect of occupational endotoxin exposure on impaired lung function and death: the Shanghai Textile Worker Study. Occup Environ Med. 2014;71(2):118–25. doi: 10.1136/oemed-2013-101676. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Card JW, Carey MA, Bradbury JA, et al. Gender differences in murine airway responsiveness and lipopolysaccharide-induced inflammation. J Immunol. 2006;177(1):621–30. doi: 10.4049/jimmunol.177.1.621. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Cui L, Gallagher LG, Ray RM, et al. Unexpected excessive chronic obstructive pulmonary disease mortality among female silk textile workers in Shanghai, China. Occup Environ Med. 2011;68(12):883–7. doi: 10.1136/oem.2010.062034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Hang J, Zhou W, Wang X, et al. Microsomal epoxide hydrolase, endotoxin, and lung function decline in cotton textile workers. American Journal of Respiratory and Critical Care Medicine. 2005;171(2):165. doi: 10.1164/rccm.200407-888OC. [DOI] [PubMed] [Google Scholar]
- 34.Bakirci N, Kalaca S, Fletcher AM, et al. Predictors of early leaving from the cotton spinning mill environment in newly hired workers. Occup Environ Med. 2006;63(2):126–30. doi: 10.1136/oem.2005.021352. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Su WL, Chen YH, Liou SH, Wu CP. Meta-analysis of standard mortality ratio in cotton textile workers. European journal of epidemiology. 2004;19(11):989–97. doi: 10.1007/s10654-004-0917-3. [DOI] [PubMed] [Google Scholar]
- 36.Mehta AJ, Wang XR, Eisen EA, et al. Work area measurements as predictors of personal exposure to endotoxin and cotton dust in the cotton textile industry. Ann Occup Hyg. 2008;52(1):45–54. doi: 10.1093/annhyg/mem061. [DOI] [PubMed] [Google Scholar]
- 37.Lane SR, Sewell RD. The bacterial profile of cotton lint from worldwide origins, and links with occupational lung disease. American journal of industrial medicine. 2007;50(1):42–7. doi: 10.1002/ajim.20412. doi: 10.1002/ajim.20412[published Online First: Epub Date]|. [DOI] [PubMed] [Google Scholar]
- 38.Cinkotai FF, Rigby A, Pickering CA, Seaborn D, Faragher E. Recent trends in the prevalence of byssinotic symptoms in the Lancashire textile industry. Br J Ind Med. 1988;45(11):782–9. doi: 10.1136/oem.45.11.782. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Ege MJ, Mayer M, Normand AC, et al. Exposure to environmental microorganisms and childhood asthma. N Engl J Med. 2011;364(8):701–9. doi: 10.1056/NEJMoa1007302. [DOI] [PubMed] [Google Scholar]
- 40.Lynch SV, Wood RA, Boushey H, et al. Effects of early-life exposure to allergens and bacteria on recurrent wheeze and atopy in urban children. J Allergy Clin Immunol. 2014;134(3):593–601. doi: 10.1016/j.jaci.2014.04.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.