Abstract
Due to their size and anatomical similarity to humans, baboons make an excellent model for reproductive studies. Baboons have a simple short cervix, muscular uterus, ovaries just lateral to the uterus, and similar vasculature to that of humans. Because of the size of the animals, instruments designed for use in women can be readily used on baboons. Noninvasive determination of phase of estrous cycle is readily made by observation of changes in perineal sexual skin turgor and color. Some advantages of use of baboons compared to other nonhuman primates is that they are non-seasonal breeders, allowing for studies to be conducted year-round, have minimal infectious disease risks to humans as they do not carry Herpes B, and have a social structure allowing for easy group formation. Baboons serve as good models for many conditions in humans and should be considered for studies investigating reproductive issues.
Keywords: baboon, nonhuman primate, gynecology, contraception, research, model, reproduction
1. Introduction
Baboons (Papio sp.) are relatively large nonhuman primates that serve as good models for reproductive studies, as their reproductive tracts are similar to that of humans [1, 2, 3, 4.] Sexually mature female baboons typically range in weight from 14 – 18 kg, but can approach 30kg if obese [5.] Baboons have a relatively long life span and sexual maturity occurs at age 4–6 [6, 7, 8.] Females naturally experience an interbirth interval of approximately one to three years [6, 9,] which allows for multiple birthing event studies to be conducted in a reasonable timeline in this closely related species.
A significant positive consideration for the use of the baboon is the safety to personnel. All macaques are considered potential carriers of Macacacine (Cercopithecine) herpesvirus 1 (Herpes B) which has a high rate of fatality when contracted by humans [10, 11, 12.] Since baboons are unable to carry this virus, handling and collection of blood and other body fluid samples involves lower risk than with macaques. Although large, baboons are relatively docile, and they can easily be trained to permit injections and observations without the need to handle the animals [8, 13.]
2. Reproductive cycles and pregnancy
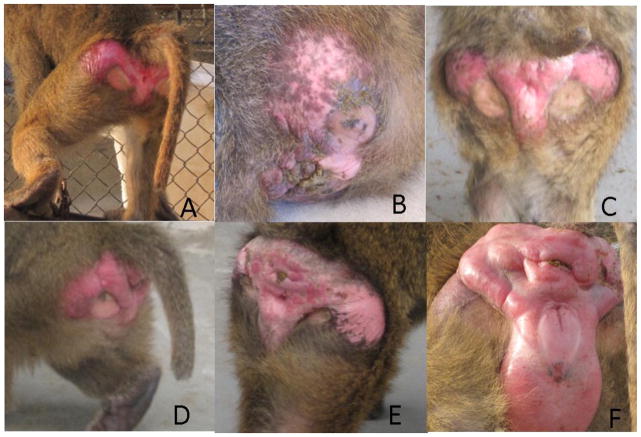
Female baboons display large perineal sexual skin swelling, which predictably changes throughout the estrous cycle and pregnancy, making determination of phase of cycle easy to distinguish visually by a trained observer. Swelling occurs in response to estrogen and is maximal at the time of ovulation, resolving in the luteal phase [6, 8, 14, 15, 16, 17] (Figure 1.) Since these changes are predictable, careful monitoring can eliminate the need for invasive procedures, such as blood collection, to determine menstrual cycle stage. However, the engorged sex skin can also make visualization of the straight cervical canal difficult due to elongation of the vaginal vault. Therefore, transcervical procedures are optimally timed during the early follicular or late luteal phase when swelling is minimal [18.] Alternatively, hormonal contraceptive agents such as medroxyprogesterone acetate or combined oral contraceptives can be used to manipulate the cycle [15, 18.] Females typically become sexually mature between 4 and 6 years of age [19, 20.] In a closed colony at the Southwest National Primate Research Center (San Antonio, TX), females are maintained in breeding groups from approximately 6 years until 16 to 18 years of age. Aged baboons can experience menopause similar to humans, making reproductive problems more difficult to diagnose after this age [8, 21, 22.] Pregnancy can be diagnosed accurately by trained personnel observing changes in perineal sexual skin, bimanual palpation, and/or the use of ultrasound [8.] Gestation length is approximately 163–185 days [6, 8, 9, 19, 23, 24, 25.] Standard biometric ultrasound measurements are available to determine gestational age and growth profiles of fetuses [26, 27, 28, 29.] Infants are born more developed than humans, but depend on their dams for care and nutrition up to and beyond weaning. Typically, the infants are carried by the dams until approximately 5 to 6 months of age when the dam will wean the infant off the breast [6, 19, 30.] The infant will still cling to the dam (even if pregnant again), or other adults after weaning, learning the normal foraging and other behaviors of the adults [7.]
Figure 1.
Perineal sexual skin swelling indicating phase of menstrual cycle. A. Score 0 and pregnant (bright pink/purple color). B. Score 0 not pregnant. C. Score 1 slight amount of swelling. D. Score 2 moderate amount of swelling. E. Score 3 significant amount of swelling. F. Score 4 fully swollen and ready to ovulate.
When pregnant, baboons have a single discoid hemochorial villous placenta [8, 31,] similar to humans. In comparison, macaques have a bi-discoid placenta and a slightly shorter gestation (168 days [6, 8, 32, 33.]) As with humans, some baboons will immediately return to cycling after delivery, and are therefore able to conceive rapidly [9.] Others do not resume cycling until the infant is weaned at 5 to 6 months [6.] Single births are typical, and twins rare but possible [6, 8, 34.] Unlike macaques which tend to be seasonal breeders, baboons are monthly ovulators with no breeding season [6, 8, 35.]
3. Social Biology
Social housing of baboons is readily achievable because they have a meld mold society which typically allows new members to join with minimal fighting and disruption to social hierarchy. This is in contrast to macaques which are highly territorial and introduction of new individuals to the group may result in violent attacks. At SNPRC, a single fertile male baboon can cover a group of 15 or more females with a high pregnancy success rate [6, 8, 13.] Many males will accept unrelated offspring in the group, but care should be exercised, as some males will commit infanticide. Baboons have a mild temperament, and they can be readily trained to run through chute systems, making movement, observation, and treatment of animals relatively simple to accomplish [8.]
4. Gynecologic procedures
Because of their relatively large size, instruments such as vaginal speculums and dilators that are used in small women can be utilized effectively with no modifications in baboons. The cervix is easily visualized and relatively short and straight, making dilation simple and rapid [3, 7, 36.] In comparison, macaques have a relatively long tortuous cervix which can make dilation and catheterization difficult and prolonged [36, 37.] Like women, baboons possess paired ovaries lateral to the uterus and a midline simplex muscular uterus which is easily identified on transabdominal ultrasound [31.] The baboon has also proven to be a useful model for embryonic stem cells and artificial reproductive technologies (ART) due to its size, temperament, and similarity to humans [13.] The angle and orientation of the uterus is slightly different in primates as compared to humans due to adaptations allowing for different modes of locomotion [31.]
The vasculature supplying the pelvic organs in the baboon (Papio sp.) and human (Homo sapiens) are similar, with two branches coming from the internal iliac [39] and several anastomoses between uterine and ovarian arteries [31.] This is an important similarity, as transabdominal ultrasound can be used to monitor the infusion of agents into the uterus and potential uptake into the vasculature through the uterine or ovarian arteries. If visualization of the ovaries is required for reproductive studies, human vaginal probes can also be used because of the large size of the animal.
5. Conclusions
Baboons have already been used on a wide variety of reproductive studies, including pregnancy [40, 41, 42, 43,] fetal development [44, 45,] pharmacokinetics of compounds in pregnancy [46, 47,] abortifactant drugs [48,]nutrient restriction and the maternal/fetal relationship [49,] genetics [50,] endometriosis [51, 52,] hormonal [14, 16, 21, 53, 54, 55, 56,] temporary contraception [57, 58,] embryonic stem cells and ART [13,] pathology [59, 60,] and permanent contraception [18.] Their temperament and similarities to human anatomy warrant their continued use for reproductive studies. Significant advantages to the use of baboons in reproductive studies include their size, anatomy and physiology, the short straight cervix that can be easily cannulated, and increased personnel safety. Studies that require uterine sampling or administration of agents can typically be conducted in a less invasive manner (transcervically rather than laparoscopically) and with a shorter procedure time than those conducted in macaques. The use of baboons has already allowed for significant contributions to the advancement of knowledge and techniques associated with various reproductive studies, and will continue to play an important role in future studies.
Acknowledgments
This investigation used resources that were supported by the Southwest National Primate Research Center grant P51 RR013986 from the National Center for Research Resources, National Institutes of Health and that are currently supported by the Office of Research Infrastructure Programs through P51 OD011133.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Honoré EK, Tardif SD. Reproductive Biology of Baboons. In: Vandeberg JL, Williams-Blangero S, Tardif SD, editors. The Baboon in Biomedical Research. Chicago: Springer; 2009. [Google Scholar]
- 2.Bell JD, Bergin IL, Schmidt K, Zochowski MK, Aronoff DM, Patton DL. Nonhuman primate models used to study pelvic inflammatory disease caused by Chlamydia trachomatis. Infect Dis Obstet Gynecol. 2011;2011:675360. doi: 10.1155/2011/675360. doi:140.1155/2011/675360. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Chai D, Cuneo S, Falconer H, Mwenda, D’Hooghe T. Olive baboon (Papio anubis anubis) as a model for intrauterine research. J Med Primatol. 2007;36:365–9. doi: 10.1111/j.1600-0684.2006.00204.x. [DOI] [PubMed] [Google Scholar]
- 4.D’Hooghe TM, Kyama CM, Chai, et al. Nonhuman primate models for translational research in endometriosis. Reprod Sci. 2009;16:152–161. doi: 10.1177/1933719108322430. [DOI] [PubMed] [Google Scholar]
- 5.Kulkarni T, Slaughter G, Ego-Osuala C, Kochunov P, Bastarrachea RA, Mattern V, Andrade M, Higgins PB, Comuzzie AG, Voruganti VS. Hyperglycemic challenge and distribution of adipose tissue in obese baboons. Int J Diabetol Vasc Disease Res. 2014;2:43–8. [PMC free article] [PubMed] [Google Scholar]
- 6.Bernacky BJ, Gibson SV, Keeling ME, Abee CR. Nonhuman Primates (Chapter 16) In: Gox JG, Anderson LC, Lowe FM, Quimby FW, editors. Laboratory Animal Medicine. 2. San Diego: Academic Press; 2002. [Google Scholar]
- 7.Onyango PO, Gesquiere LR, Altmann J, Alberts SC. Puberty and dispersal in a wild primate population. Horm Behav. 2013:240–9. doi: 10.1016/j.yhbeh.2013.02.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Tardif S, Carville A, Elmore D, Williams LE, Rice K. Reproduction and Breeding of Nonhuman Primates (Chapter 8) In: Abee CR, Mansfield K, Tardif S, Morris T, editors. Nonhuman Primates in Biomedical Research Volume 1: Biology and Management. 2. San Diego: Academic Press; 2012. [Google Scholar]
- 9.Altmann J, Altmann SA, Hausfater G. Primate infant’s effects on mother’s future reproduction. Science. 1978;201:1028–30. doi: 10.1126/science.98844. [DOI] [PubMed] [Google Scholar]
- 10.Cohen JI, Davenport DS, Stewart JA, Deitchman S, Hilliard JK, Chapman LE B Virus Working Group. Recommendations for prevention of and therapy for exposure to B Virus (Cercopithecine Herpesvirus 1) CID. 2002:1191–1203. doi: 10.1086/344754. [DOI] [PubMed] [Google Scholar]
- 11.CDC. [Accessed: 19 May 2015];B Virus (herpes B, monkey B virus, herpesvirus simiae, and herpesvirus B) http://www.cdc.gov/herpesbvirus/index.html.
- 12.Hilliard J. Monkey B virus (Chapter 57) In: Arvin A, Campadelli-Fiume G, Mocarski E, Moore PS, Roizman B, Whitely R, Yamanashi K, editors. Human Herpesviruses: Biology, Therapy, and Immunoprophylaxis. Cambridge: Cambridge University Press; 2007. [PubMed] [Google Scholar]
- 13.Simerly CR, Castro CA, Jacoby E, Grund K, Turpin J, McFarland D, Champagne J, Jimenez JB, Jr, Frost P, Bauer C, Hewitson L, Schatten G. Assisted Reproductive Technologies (ART) with baboons generate live offspring: a nonhuman primate model for ART and reproductive sciences. Reprod Sci. 2010;17:917–30. doi: 10.1177/1933719110374114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Gauthier CA. Reproductive parameters and paracallosal skin color changes in captive female guinea baboons, Papio papio. Am J Primatol. 1999;47:67–74. doi: 10.1002/(SICI)1098-2345(1999)47:1<67::AID-AJP8>3.0.CO;2-#. [DOI] [PubMed] [Google Scholar]
- 15.Guy AJ, Schuerch FS, Heffernan S, Thomson PC, O’Brien JK, McGreevy PD. The effect of medroxyprogesterone acetate on behavioural responses of captive female hamadryas baboons (Papio hamadryas) Anim Reprod Sci. 2008;108:412–24. doi: 10.1016/j.anireprosci.2007.09.008. [DOI] [PubMed] [Google Scholar]
- 16.Wildt DE, Doyle LL, Stone SC, Harrison RM. Correlation of perineal swelling with serum ovarian hormone levels, vaginal cytology, and ovarian follicular development during the baboon reproductive cycle. Primates. 1977;18:261–70. [Google Scholar]
- 17.Shaikh A, Shaikh S, Celaya C, Goldzieher J. Ovulation pattern in successive cycles in the baboon. Primates. 1982;23:592–5. [Google Scholar]
- 18.Jensen JT, Hanna C, Yao S, Bauer C, Hergert C, Slayden OD. Effect of Polidocanol Foam Concentration on Success of Tubal Occlusion Following Transcervical Administration in Baboons. American Society for Reproductive Medicine Annual Meeting; October 18–22, 2014. [Google Scholar]
- 19.Birrell AM, Hennessy A, Gillin A, Horvath J, Tiller D. Reproductive and neonatal outcomes in captive bred baboons (Papio hamadryas) J Med Primatol. 1996;25:287–93. doi: 10.1111/j.1600-0684.1996.tb00212.x. [DOI] [PubMed] [Google Scholar]
- 20.Sigg H, Stolba A, Abegglen JJ, Dasser V. Life history of hamadryas baboons: physical development, infant mortality, reproductive parameters and family relationships. Primates. 1982;23:473–87. [Google Scholar]
- 21.Martin LJ, Carey KD, Comuzzie AG. Variation in menstrual cycle length and cessation of menstruation in captive raised baboons. Mech Aging Dev. 2003;124:865–71. doi: 10.1016/s0047-6374(03)00134-9. [DOI] [PubMed] [Google Scholar]
- 22.Packer C, Tater M, Collins A. Reproductive cessation in female mammals. Nature. 1998;392:807–11. doi: 10.1038/33910. [DOI] [PubMed] [Google Scholar]
- 23.Bercovitch FB, Harding RSO. Annual birth patterns of savanna baboons (Papio cynocephalus anubis) over a ten-year period at Gilgil, Kenya. Folia Primatol. 1994;61:115–22. doi: 10.1159/000156738. [DOI] [PubMed] [Google Scholar]
- 24.Brandt EM, Mitchell G. Parturition in primates: behavior related to birth. In: Rosenblum LA, editor. Primate Behavior Developments in Field and Laboratory Research. Vol. 2. New York: Academic Press; 1971. [Google Scholar]
- 25.Nash LT. Parturition in a feral baboon (Papio anubis) Primates. 1974;15:279–85. [Google Scholar]
- 26.Farine D, MacCarter GD, Timor-Tritch IE, Yeh MN, Stark RI. Real-time ultrasonic evaluation of baboon pregnancy: biometric measurements. J Med Primatol. 1988;17:215–21. [PubMed] [Google Scholar]
- 27.Herring JM, Fortman JD, Anderson RJ, Bennett BT. Ultrasonic determination of fetal parameters in baboons (Papio anubis) Lab Anim Sci. 1991;41:602–5. [PubMed] [Google Scholar]
- 28.Devonald KJ, Harewood WJ, Ellwood DA, Phippard AF. Fetal ultrasonic normal biometric ranges in the baboon (Papio hamadryas) J Med Primatol. 1996;25:339–45. doi: 10.1111/j.1600-0684.1996.tb00026.x. [DOI] [PubMed] [Google Scholar]
- 29.Santolaya-Forgas J, De Leon-Luis J, Friel LA, Wolf RA. Application of Carnegie stages of development to unify human and baboon ultrasound findings early in pregnancy. Ultrasound Med Biol. 2007;33:1400–5. doi: 10.1016/j.ultrasmedbio.2007.03.005. [DOI] [PubMed] [Google Scholar]
- 30.Altmann SA, Hausfater G, McCuskey SA. Life history of yellow baboons: physical development, reproductive parameters, and infant mortality. Primates. 1977;18:315–30. [Google Scholar]
- 31.Turnquist JE, Minugh-Purvis N. Nonhuman Primates. In: Abee CR, Mansfield K, Tardif S, Morris T, editors. Biomedical Research Volume 1: Biology and Management. Waltham: Elsevier; 2012. [Google Scholar]
- 32.Jacobson HN, Windle WF. Observations on mating, gestation, birth and postnatal development of Macaca mulatta. Biol. Neonat. 1960;2:105–20. doi: 10.1159/000239792. [DOI] [PubMed] [Google Scholar]
- 33.Stolte LAM. Pregnancy in the rhesus monkey. In: Bourne GH, editor. The Rhesus Monkey. Vol. 2. New York: academic Press; 1975. [Google Scholar]
- 34.Mitchell G, Brandt EM. Behavior of the female rhesus monkey during birth. In: Bourne GH, editor. The Rhesus Monkey. Vol. 2. Academic Press; New York: 1975. [Google Scholar]
- 35.Altmann J, Gesquiere L, Galbany J, Pnyango PO, Alberts SC. Life history context of reproductive aging in a wild primate model. Ann N Y Acad Sci. 2010:127–38. doi: 10.1111/j.1749-6632.2010.05531.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Hafez ESE, Jaszczak S. Comparative anatomy and histology of the cervis uteri in non-human primates. Primates. 1972;13:297–316. [Google Scholar]
- 37.Goodeaux LL, Anzalone CA, Webre MK, Graves KH, Voelkel SA. Nonsurgical technique for flushing the Macaca mulatta uterus. J Med Primatol. 1990;19:59–67. [PubMed] [Google Scholar]
- 38.Jayaprakash D, Satish KS, Ramachandra SG, Ramesh V, Seshagiri PB. Successful recovery of preimplantation embryos by nonsurgical uterine flushing in the bonnet macaque. Theriogenology. 1997;47:1019–26. doi: 10.1016/s0093-691x(97)00059-9. [DOI] [PubMed] [Google Scholar]
- 39.Swindler DR, Wood CD. An Atlas of Primate Gross Anatomy: Baboon, Chimpanzee, and Man. Malabar FL: Robert E. Krieger Publishing Company, Inc; 1973. [Google Scholar]
- 40.Eddy CA, Turner TT, Kraemer DC, Pauerstein CJ. Pattern and duration of ovum transport in the baboon (Papio anubis) Obstet Gynecol. 1976;47:658–64. [PubMed] [Google Scholar]
- 41.Enders AC, Lantz KC, Peterson PE, Hendrickx AG. From blastocyst to placenta: the morphology o fimplantation in the baboon. Hum Reprod Update. 1997;3:561–73. doi: 10.1093/humupd/3.6.561. [DOI] [PubMed] [Google Scholar]
- 42.Nyachieo A, Chai DC, Deprest J, Mwenda JM, D’Hooghe TM. The baboon as a research model for the study of endometrial biology, uterine receptivity and embryo implantation. Gynecol Obstet Invest. 2007;64:149–55. doi: 10.1159/000101739. Epub 2007 Oct 4. [DOI] [PubMed] [Google Scholar]
- 43.Schlabritz-Loutsevitch N, Moore CM, Lopez-Alvarenga JC, Dunn BG, Dudley D, Hubbard GB. The baboon model (Papio hamadryas) of fetal loss: maternal weight, age, reproductive history and pregnancy outcome. J Med Primatol. 2008;37:337–45. doi: 10.1111/j.1600-0684.2008.00297.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Blanco CL, Moreira AG, McGill-Vargas LL, Anzueto DG, Nathanielsz P, Musi N. Antenatal corticosteroids alter insulin signaling pathways in fetal baboon skeletal muscle. J Endocrinol. 2014;221(2):253–60. doi: 10.1530/JOE-13-0504. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Houston ML. the development of the baboon (Papio sp.) placenta during the fetal period of gestation. Am J Anat. 1969;126:17–29. doi: 10.1002/aja.1001260103. [DOI] [PubMed] [Google Scholar]
- 46.Rytting E, Wang X, Vernikovskaya DI, Zhan Y, Bauer C, Abdel-Rahman SM, Ahmed MS, Nanovskaya TN. Metabolism and disposition of bupropion in pregnant baboons (Papio cynocephalus) Drug Metab Dispos. 2014;42:1773–9. doi: 10.1124/dmd.114.058255. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Wang X, Paul JA, Nanovskaya TN, Hankins GD, Ahmed MS. Quantitative determination of telavancin in pregnant baboon plasma by solid-phase extraction and LC-ESI-MS. J Pharm Biomed Anal. 2014;98:107–12. doi: 10.1016/j.jpba.2014.04.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Beck LR, Pope VZ. Demonstration of an early abortifacient effect of norethisterone (NET) in the primate (baboon) Contraception. 1982:97–105. doi: 10.1016/0010-7824(82)90022-1. [DOI] [PubMed] [Google Scholar]
- 49.Cox LA, Li C, Glenn JP, Lange K, Spradling KD, Nathanielsz PW, Jansson T. Expression of the transcriptome in maternal nutrient reduction in baboons is dependent on fetal sex. J Nutr. 2013;143:1698–708. doi: 10.3945/jn.112.172148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Cox LA, Comuzzie AG, Havill LM, Karere GM, Spradling KD, Mahaney MC, Nathanielsz PW, Nicolella DP, Shade RE, Voruganti S, VandeBerg JL. Baboons as a model to study genetics and epigenetics of human disease. ILAR. 2013;54:106–21. doi: 10.1093/ilar/ilt038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.D’Hooghe TM. Clinical relevance of the baboon as a model for the study of endometriosis. Fertil Steril. 1997;68:613–25. doi: 10.1016/s0015-0282(97)00277-x. [DOI] [PubMed] [Google Scholar]
- 52.Kyama CM, Mihalyi A, Chai D, Simsa P, Mwenda JM, D’Hooghe TM. Baboon model for the study of endometriosis. Womens Health (Lond Engl) 2007;3:637–46. doi: 10.2217/17455057.3.5.637. [DOI] [PubMed] [Google Scholar]
- 53.Caperton L, Eddy C, Leland MM, Carey KD, McCarrey JR. Alteration of the menstrual cycle in baboons placed on tethering devices and moved to individual housing – a stress model for a follicular phase defect. J Med Primatol. 2006;35:341–5. doi: 10.1111/j.1600-0684.2006.00185.x. [DOI] [PubMed] [Google Scholar]
- 54.Castracane VD, Hendrickx AG, Henson MC. Serum leptin in nonpregnant and pregnant women and in old and new world nonhuman primates. Exp Biol Med (Maywood) 2005;230:251–4. doi: 10.1177/153537020523000404. [DOI] [PubMed] [Google Scholar]
- 55.Cary ME, Valentine B, White GL. The effects of confinement environment on reproductive efficiency in the baboon. Contemp Top Lab Animal Sci. 2003;42:35–9. [PubMed] [Google Scholar]
- 56.Goncharov N, Aso T, Cekan Z, Pachalia N, Diczfalusy E. Hormonal changes during the menstrual cycle of the baboon (Papio hamadryas) Acta Endocrinol (Copenh) 1976;82:396–412. doi: 10.1530/acta.0.0820396. [DOI] [PubMed] [Google Scholar]
- 57.Bell JD, Bergin IL, Natavio MF, et al. Feasibility of LNG-IUS in a baboon model. Contraception. 2013;87:380–4. doi: 10.1016/j.contraception.2012.08.032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Hashway SA, Bergin IL, Bassis CM, et al. Impact of a hormone-releasing intrauterine system on the vaginal microbiome: a prospective baboon model. Journal of medical primatology. 2014;43:89–99. doi: 10.1111/jmp.12090. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Bommineni YR, Dick EJ, Jr, Malapati AR, Owston MA, Hubbard GB. Natural pathology of the baboon (Papio sp.) J Med Primatol. 2011;40:142–55. doi: 10.1111/j.1600-0684.2010.00463.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Dick EJ, Jr, Owston MA, David JM, Sharp RM, Rouse S, Hubbard GB. Mortality in captive baboons (Papio sp.): a 23 year study. J Med Primatol. 2014;43:169–96. doi: 10.1111/jmp.12101. [DOI] [PMC free article] [PubMed] [Google Scholar]