FIGURE 6. Role for CysK during bacterial contact-dependent growth inhibition (CDI).
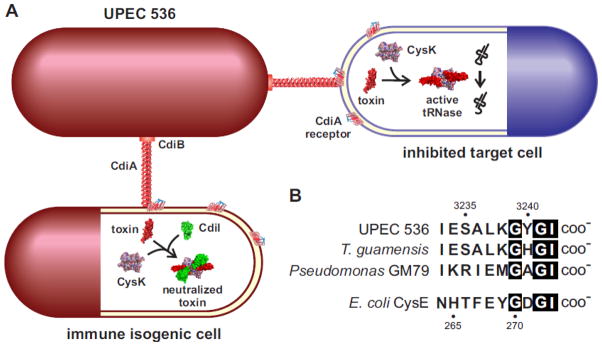
A. The CDI system from uropathogenic E. coli 536 (UPEC 536) deploys a CysK-dependent nuclease. CdiB secretes and assembles the filamentous CdiA protein on the surface of UPEC 536 cells. CdiA binds to a receptor on other E. coli cells and delivers a toxin domain derived from its C-terminus. The delivered toxin binds specifically to CysK in target cells, thereby activating the nuclease to cleave tRNA molecules. UPEC 536 also deliver toxins to sibling cells, but the toxin complex is inactivated by the CdiI immunity protein. The resulting ternary complex has no tRNase activity and isogenic sibling cells are not inhibited.
B. CdiA proteins from UPEC 536 and other bacteria carry a C-terminal peptide motif that resembles the C-terminus of CysE. The C-terminal sequences of CdiA proteins from UPEC 536 (Uniprot: Q0T963), Trabulsiella guamensis ATCC 49490 (A0A085AAB6) and Pseudomonas sp. GM79 (J3D0E2) are presented. Amino acid sequences are given in one-letter code and residue numbering corresponds to CdiAUPEC536 (top) and E. coli CysE (bottom).
